In the previous section, we completed the discussion on the Bohr model electronic configuration of atoms. We also saw isotopes. In this section, we will learn about Chemical bonding.
We have seen how the electrons are arranged in the various shells. Each element has a unique arrangement of electrons. This arrangement is called the configuration. We have learned to write the configuration of a large number of elements.
Now we consider the number of electrons in the outer most shell of an atom. Note that, the outer most shell is what matters for our present discussion. We want the number of electrons in this outer most shell.
If the number of electrons in the outer most shell is '8', then it is called an Octet electron configuration. Neon and Argon are good examples. They are shown in the fig. 2.1 below:
Elements, whose atoms have octet electron configuration, generally will not take part in chemical reactions. This is because, it is a stable configuration. So what about those elements which do not have this octet?
They will try to attain the octet, so that, there will be stability. Let us see how this octet can be attained:
• We will take the example of Sodium. It has a configuration of 2, 8, 1. (see fig.3.2 below)
• The outer most shell has 1 electron. So it needs 7 more electrons to attain octet
• Another example is Magnesium. It has a configuration of 2, 8, 2. (see fig.3.2 below)
• The outer most shell has 2 electrons. So it needs 6 more electrons to attain octet
• Yet another example is Chlorine. It has a configuration of 2, 8, 7. (see fig.3.2 below)
• The outer most shell has 7 electrons. So it needs 1 more electron to attain octet
There are many such elements which are in 'need of electrons' to attain octet. How do they get the required number of electrons?
■ Consider the example of sodium and chlorine.
• We have seen the configuration of sodium above
• It has 1 electron in the outer most shell. So it needs 7 more electrons to attain octet
• It is easier to 'lose the 1 electron', than to 'obtain 7 electrons'
• When the one electron in the M-shell is taken away, the M-shell as a whole will disappear
• The L-shell will then become the 'outer most shell'
• The L-shell already have 8 electrons. So when L-shell becomes the outer most shell, it is an octet
• Now consider chlorine. We have seen it's configuration above
• It has 7 electrons in it's outer most shell. So it needs one more electron to attain octet
[Another way to attain octet is to lose the 7 electrons in the outer most shell. But it is easier to accept one electron than to lose 7 electrons]
• So it will accept one electron from the sodium atom that we saw above
When this donation and acceptance takes place, some other important changes also occurs:
• Normally, an atom is neutral because, the number of protons is equal to the number of electrons
• But when one electron is donated, the number of protons become excess. It becomes excess by '1'
• So the sodium atom which was initially neutral, has now become 'charged'
• We call such charged particles as ions. When an atom has become charged, we can no longer call it an 'atom'. We call it an 'ion'
• So our 'sodium atom' has become a 'sodium ion'. And this sodium ion has a positive charge
• Similarly, the chlorine atom has accepted an electron
• Number of electrons have become excess by 1
• It has now become a chlorine ion. And this chlorine ion has a negative charge
• So we see that the atoms have become ions
• Sodium atom has become sodium ion, and chlorine atom has become chlorine ion
• An electrostatic force of attraction will develop between the two ions. This force develops because, they are 'oppositely charged'
• This force will hold the two ions together
• Thus a molecule consisting of one ion of sodium and one ion of chlorine will be formed
■ This molecule is the 'molecule of sodium chloride'. It is represented as NaCl
The transfer of electron between sodium and chlorine can be represented pictorially as shown in the fig. 3.3 below:
• Consider the left side of the arrow: On either sides of the '+' sign, we have bohr models of sodium and chlorine. This shows the initial state
• Sodium has one electron in the outer most shell. On the right side of the arrow, this atom of sodium has become an 'ion'. The outer most electron is lost. So the bohr model is shown inside square brackets, and a '+' sign is given at top right. This indicates one positive charge
• On the left side of the arrow, the bohr model of the chlorine atom has 7 electrons in the outer most shell. On the right side, the number of electrons in the outer most shell becomes 8. The chlorine atom becomes a chlorine ion. The model is put inside square brackets, and a '-' sign is given at top right. This indicates one negative charge
• Each entity inside square brackets is an ion. These 'oppositely charged' ions are held together by electrostatic force of attraction. So the diagram gives us a clear picture of how sodium and chlorine attain octet. It also shows us the formation of sodium chloride
Another method to represent the above process, is by using Electron dot diagram. In this method, only those electrons in the outer most shell are shown. This is because, they are the only electrons taking part in the bond formation. The dot diagram showing the formation of sodium chloride is shown in fig.3.4 below:
• The outer most electrons are shown as dots around the symbol of the atom
• By counting the number of dots, we can see that, sodium has lost one electron, and chlorine has gained one electron
• The initial and final configurations are also given. Sodium and chlorine has attained octet
• Sodium has become a positive ion. This is due to the loss of electron. One electron is lost. so the charge is 1+. For a charge of magnitude one, the '1' is not written. So we just write '+' on the top right
• Chlorine has become a negative ion. This is due to the gain of electron. One electron is gained. so the charge is 1-. For a charge of magnitude one, the '1' is not written. So we just write '-' on the top right
• The 'oppositely charged' ions are held together by electrostatic force of attraction. Thus a molecule NaCl of sodium chloride is formed
Formation of Magnesium oxide
• Magnesium has a configuration 2, 8, 2. (See here). So it has 2 electrons in the outer most shell
• It needs 6 more electrons to attain octet. It is easier to lose the 2 electrons than to obtain 6 electrons
• Oxygen has a configuration 2, 6. So it has 6 electrons in the outer most shell
• It needs 2 more electrons to attain octet. So it will accept the 2 electrons which are donated by magnesium
• With the above information, we can draw the electron dot diagram as shown in fig.3.5 below:
• The outer most electrons are shown as dots around the symbol of the atom
• By counting the number of dots, we can see that, magnesium has lost two electrons, and oxygen has gained those two electrons
• The initial and final configurations are also given. Magnesium and oxygen has attained octet
• Magnesium has become a positive ion. This is due to the loss of electrons. Two electrons are lost. So the charge is 2+. So we write '2+' on the top right
• Chlorine has become a negative ion. This is due to the gain of electron. Two electrons are gained. So the charge is 2-. So we write '2-' on the top right
• The 'oppositely charged' ions are held together by electrostatic force of attraction. Thus a molecule MgO of Magnesium oxide is formed
Formation of sodium oxide
• Na has a configuration 2, 8, 1. So it has 1 electron in the outer most shell
• It needs 7 more electrons to attain octet. It is easier to lose the 1 electron than to obtain 7 electrons
• O has a configuration 2, 6. So it has 6 electrons in the outer most shell
• It needs 2 more electron to attain octet.
• So 2 atoms of Na will combine with one atom of O. So that:
♦ One Na will donate one electron to O
♦ The other Na will also donate one electron to O
♦ So, in total, O gets two electrons
• With the above information, we can draw the electron dot diagram as shown in fig.3.6 below:
• The outer most electrons are shown as dots around the symbol of the atom
• By counting the number of dots, we can see that, each Na has lost one electron, and the O atom have gained those two electrons. (one electron from each Na)
• The initial and final configurations are also given. Na and O have attained octet
• Na has become a positive ion. This is due to the loss of electron. One electron is lost. So the charge is 1+. So we write '+' on the top right
• O has become a negative ion. This is due to the gain of electron. Two electrons are gained by O. So the charge is 2- . So we write '-' on the top right
• The 'oppositely charged' ions are held together by electrostatic force of attraction. Thus a molecule Na2O is formed
In the next section, we will see some solved examples. We will also learn about Cations and Anions.
We have seen how the electrons are arranged in the various shells. Each element has a unique arrangement of electrons. This arrangement is called the configuration. We have learned to write the configuration of a large number of elements.
Now we consider the number of electrons in the outer most shell of an atom. Note that, the outer most shell is what matters for our present discussion. We want the number of electrons in this outer most shell.
If the number of electrons in the outer most shell is '8', then it is called an Octet electron configuration. Neon and Argon are good examples. They are shown in the fig. 2.1 below:
 |
| Fig.3.1 |
They will try to attain the octet, so that, there will be stability. Let us see how this octet can be attained:
• We will take the example of Sodium. It has a configuration of 2, 8, 1. (see fig.3.2 below)
• The outer most shell has 1 electron. So it needs 7 more electrons to attain octet
• Another example is Magnesium. It has a configuration of 2, 8, 2. (see fig.3.2 below)
• The outer most shell has 2 electrons. So it needs 6 more electrons to attain octet
• Yet another example is Chlorine. It has a configuration of 2, 8, 7. (see fig.3.2 below)
• The outer most shell has 7 electrons. So it needs 1 more electron to attain octet
There are many such elements which are in 'need of electrons' to attain octet. How do they get the required number of electrons?
■ Consider the example of sodium and chlorine.
• We have seen the configuration of sodium above
• It has 1 electron in the outer most shell. So it needs 7 more electrons to attain octet
• It is easier to 'lose the 1 electron', than to 'obtain 7 electrons'
• When the one electron in the M-shell is taken away, the M-shell as a whole will disappear
• The L-shell will then become the 'outer most shell'
• The L-shell already have 8 electrons. So when L-shell becomes the outer most shell, it is an octet
• Now consider chlorine. We have seen it's configuration above
• It has 7 electrons in it's outer most shell. So it needs one more electron to attain octet
[Another way to attain octet is to lose the 7 electrons in the outer most shell. But it is easier to accept one electron than to lose 7 electrons]
• So it will accept one electron from the sodium atom that we saw above
When this donation and acceptance takes place, some other important changes also occurs:
• Normally, an atom is neutral because, the number of protons is equal to the number of electrons
• But when one electron is donated, the number of protons become excess. It becomes excess by '1'
• So the sodium atom which was initially neutral, has now become 'charged'
• We call such charged particles as ions. When an atom has become charged, we can no longer call it an 'atom'. We call it an 'ion'
• So our 'sodium atom' has become a 'sodium ion'. And this sodium ion has a positive charge
• Similarly, the chlorine atom has accepted an electron
• Number of electrons have become excess by 1
• It has now become a chlorine ion. And this chlorine ion has a negative charge
• So we see that the atoms have become ions
• Sodium atom has become sodium ion, and chlorine atom has become chlorine ion
• An electrostatic force of attraction will develop between the two ions. This force develops because, they are 'oppositely charged'
• This force will hold the two ions together
• Thus a molecule consisting of one ion of sodium and one ion of chlorine will be formed
■ This molecule is the 'molecule of sodium chloride'. It is represented as NaCl
The transfer of electron between sodium and chlorine can be represented pictorially as shown in the fig. 3.3 below:
 |
| Fig.3.3 |
• Sodium has one electron in the outer most shell. On the right side of the arrow, this atom of sodium has become an 'ion'. The outer most electron is lost. So the bohr model is shown inside square brackets, and a '+' sign is given at top right. This indicates one positive charge
• On the left side of the arrow, the bohr model of the chlorine atom has 7 electrons in the outer most shell. On the right side, the number of electrons in the outer most shell becomes 8. The chlorine atom becomes a chlorine ion. The model is put inside square brackets, and a '-' sign is given at top right. This indicates one negative charge
• Each entity inside square brackets is an ion. These 'oppositely charged' ions are held together by electrostatic force of attraction. So the diagram gives us a clear picture of how sodium and chlorine attain octet. It also shows us the formation of sodium chloride
Another method to represent the above process, is by using Electron dot diagram. In this method, only those electrons in the outer most shell are shown. This is because, they are the only electrons taking part in the bond formation. The dot diagram showing the formation of sodium chloride is shown in fig.3.4 below:
 |
| Fig.3.4 |
• By counting the number of dots, we can see that, sodium has lost one electron, and chlorine has gained one electron
• The initial and final configurations are also given. Sodium and chlorine has attained octet
• Sodium has become a positive ion. This is due to the loss of electron. One electron is lost. so the charge is 1+. For a charge of magnitude one, the '1' is not written. So we just write '+' on the top right
• Chlorine has become a negative ion. This is due to the gain of electron. One electron is gained. so the charge is 1-. For a charge of magnitude one, the '1' is not written. So we just write '-' on the top right
• The 'oppositely charged' ions are held together by electrostatic force of attraction. Thus a molecule NaCl of sodium chloride is formed
Formation of Magnesium oxide
• Magnesium has a configuration 2, 8, 2. (See here). So it has 2 electrons in the outer most shell
• It needs 6 more electrons to attain octet. It is easier to lose the 2 electrons than to obtain 6 electrons
• Oxygen has a configuration 2, 6. So it has 6 electrons in the outer most shell
• It needs 2 more electrons to attain octet. So it will accept the 2 electrons which are donated by magnesium
• With the above information, we can draw the electron dot diagram as shown in fig.3.5 below:
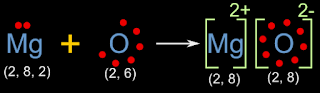 |
| Fig.3.5 |
• By counting the number of dots, we can see that, magnesium has lost two electrons, and oxygen has gained those two electrons
• The initial and final configurations are also given. Magnesium and oxygen has attained octet
• Magnesium has become a positive ion. This is due to the loss of electrons. Two electrons are lost. So the charge is 2+. So we write '2+' on the top right
• Chlorine has become a negative ion. This is due to the gain of electron. Two electrons are gained. So the charge is 2-. So we write '2-' on the top right
• The 'oppositely charged' ions are held together by electrostatic force of attraction. Thus a molecule MgO of Magnesium oxide is formed
Formation of sodium oxide
• Na has a configuration 2, 8, 1. So it has 1 electron in the outer most shell
• It needs 7 more electrons to attain octet. It is easier to lose the 1 electron than to obtain 7 electrons
• O has a configuration 2, 6. So it has 6 electrons in the outer most shell
• It needs 2 more electron to attain octet.
• So 2 atoms of Na will combine with one atom of O. So that:
♦ One Na will donate one electron to O
♦ The other Na will also donate one electron to O
♦ So, in total, O gets two electrons
• With the above information, we can draw the electron dot diagram as shown in fig.3.6 below:
 |
| Fig.3.6 |
• By counting the number of dots, we can see that, each Na has lost one electron, and the O atom have gained those two electrons. (one electron from each Na)
• The initial and final configurations are also given. Na and O have attained octet
• Na has become a positive ion. This is due to the loss of electron. One electron is lost. So the charge is 1+. So we write '+' on the top right
• O has become a negative ion. This is due to the gain of electron. Two electrons are gained by O. So the charge is 2- . So we write '-' on the top right
• The 'oppositely charged' ions are held together by electrostatic force of attraction. Thus a molecule Na2O is formed
In the next section, we will see some solved examples. We will also learn about Cations and Anions.

No comments:
Post a Comment