In the previous section, we have seen that electricity can be produced from chemical reactions. Is the reverse possible? That is., Can we make a reaction to occur by passing electricity? In this section, we will see the answer.
1. Take some solid cupric chloride (CuCl2) in a beaker.
2. Take two graphite rods. Bring them into contact with the cupric chloride. But the rods should not touch each other.
3. Connect one rod to the positive terminal of the battery. Connect the other rod to the negative terminal through a volt meter.
4. We can see that there is no passage of current. That means, solid cupric chloride does not conduct electricity.
Now let us modify the experiment:
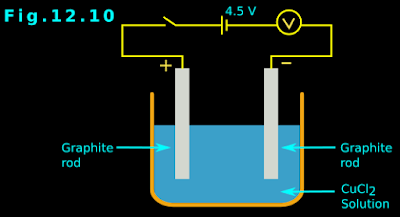
• Make the cupric chloride into a solution by adding water. The rest of the arrangements are the same. This is shown in fig.12.10 below:
• In this arrangement, the voltmeter will show that current is passing through the circuit.
So how does aqueous solution of cupric chloride conduct electricity?
We will write the answer in steps:
1. When solid CuCl2 is made into an aqueous solution, it dissociates into Cu+2 and Cl-1 ions.
• So an aqueous solution of CuCl2 is actually a mixture of Cu+2 and Cl-1 ions.
2. When the switch is turned on, the electrons begin to flow from the negative terminal of the battery to the positive terminal.
• For that, the electrons first reach the graphite rod which is connected to the negative terminal of the battery. That rod becomes negatively charged.
• So we can say that the rod connected to the negative terminal of the battery is the negative electrode in this experiment.
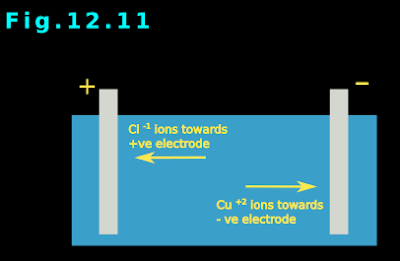
3. The positive Cu+2 ions, present in the solution will move towards this negative electrode. See fig.12.11 below:
• Each Cu+2 ion will receive two electrons, and they will become Cu atom. Let us write the equation:
Cu+2 (aq) + 2e-1 ⟶ Cu0 (s)
• This is a reduction reaction. Because, the oxidation number of Cu is reduced from +2 to zero.
• In other words:
Gaining electrons is reduction. So this is a reduction reaction
• The newly formed copper atoms stick to the surface of the electrode.
• Also, the number of Cu+2 ions present in the solution will go on decreasing. So the blue colour of the solution (which is due to the presence of Cu+2 ions) will fade
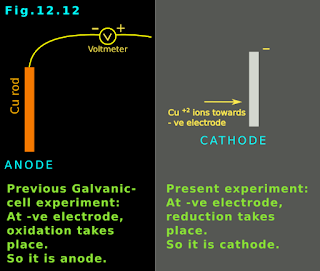
• We have seen earlier (when we discussed galvanic cells) that, the electrode at which reduction takes place is the cathode.
• So in the present experiment, the negative electrode is the cathode.
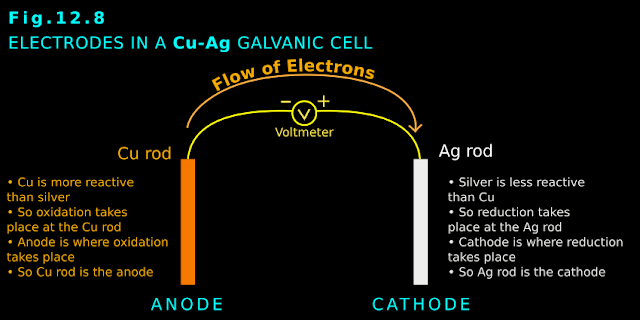
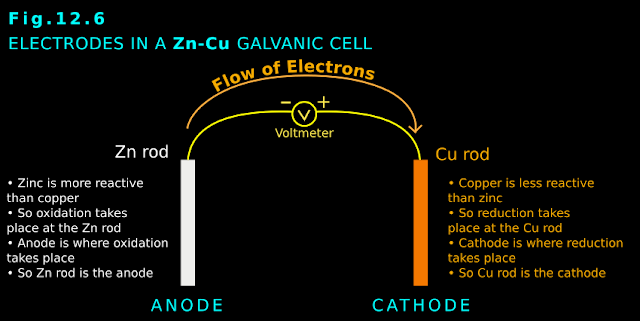
[Recall that, in the galvanic cell, the negative electrode was the anode. Because there, oxidation takes place at the negative electrode].
■ It is interesting to note the following:
• In the present experiment: Negative electrode ⟶ reduction takes place ⟶ cathode
• In galvanic cell: Negative electrode ⟶ oxidation takes place ⟶ anode
This is shown in the fig.12.12 below:
■ Note:
• An electrode may be positive or negative
♦ If oxidation takes place at that electrode, it is the anode
♦ If reduction takes place at that electrode, it is the cathode
4. Now let us continue our discussion:
• The negatively charged Cl-1 ions in the solution move towards the positive electrode. See fig.12.11 above.
• They donate their electrons to that electrode. Thus the circuit is completed. Let us write the equation:
2Cl-1 (aq) ⟶ 2Cl 0 (s) + 2e-1
• This is a oxidation reaction. Because the oxidation number of Cl increases from -1 to zero.
In other words:
Losing electrons is oxidation. So this is an oxidation reaction.
• So chlorine is formed at this electrode. They come out as bubbles from near this electrode.
• We have seen that, the electrode at which oxidation takes place is the anode.
• So in the present experiment, the positive electrode is the anode. [Recall that, in the galvanic cell, the positive electrode was the cathode. Because there, reduction takes place at the positive electrode].
■ It is interesting to note the following:
• In the present experiment: Positive electrode ⟶ oxidation takes place ⟶ anode
• In galvanic cell: Positive electrode ⟶ reduction takes place ⟶ cathode
This is shown in fig.12.13 below:
5. From (3) and (4) we can say that:
■ Both in the present reaction and in the previous galvanic cell,
• The electrode at which oxidation takes place is the anode
• The electrode at which reduction takes place is the cathode
■ But positive and negative electrodes are interchanged:
• In the present reaction, anode is the positive electrode and cathode is the negative electrode
• In the previous galvanic cell, anode is the negative electrode and cathode is the positive electrode
♦ In solid state, ions are held firmly in position. They cannot move.
• This information tells us about another possibility:
If the cupric chloride is heated to a molten state, then also, the ions will acquire mobility. So the circuit will be completed.
• That means, instead of aqueous solution, we can use the molten state of the salt also, for passing electricity.
■ Electrolytes are substances which conduct electricity in molten states or in aqueous solutions and undergo a chemical change. Acids, alkalies and salts are electrolytes in their molten state or in aqueous solution.
■ The process of chemical change taking place in an electrolyte by passing electricity is called electrolysis
■ So now we can define a new type of cell: The electrolytic cell:
In an electrolytic cell, external electricity is allowed to pass through an electrolyte through two electrodes, as a result of which, a chemical reaction takes place in the electrolyte.
• In other words, an electrolytic cell is an apparatus used to carry out electrolysis
The following table 12.2 shows the difference between Galvanic cell and Electrolytic cell
In the next section, we will see electrolysis of water.
1. Take some solid cupric chloride (CuCl2) in a beaker.
2. Take two graphite rods. Bring them into contact with the cupric chloride. But the rods should not touch each other.
3. Connect one rod to the positive terminal of the battery. Connect the other rod to the negative terminal through a volt meter.
4. We can see that there is no passage of current. That means, solid cupric chloride does not conduct electricity.
• Make the cupric chloride into a solution by adding water. The rest of the arrangements are the same. This is shown in fig.12.10 below:
• In this arrangement, the voltmeter will show that current is passing through the circuit.
So how does aqueous solution of cupric chloride conduct electricity?
We will write the answer in steps:
1. When solid CuCl2 is made into an aqueous solution, it dissociates into Cu+2 and Cl-1 ions.
• So an aqueous solution of CuCl2 is actually a mixture of Cu+2 and Cl-1 ions.
2. When the switch is turned on, the electrons begin to flow from the negative terminal of the battery to the positive terminal.
• For that, the electrons first reach the graphite rod which is connected to the negative terminal of the battery. That rod becomes negatively charged.
• So we can say that the rod connected to the negative terminal of the battery is the negative electrode in this experiment.
3. The positive Cu+2 ions, present in the solution will move towards this negative electrode. See fig.12.11 below:
• Each Cu+2 ion will receive two electrons, and they will become Cu atom. Let us write the equation:
Cu+2 (aq) + 2e-1 ⟶ Cu0 (s)
• This is a reduction reaction. Because, the oxidation number of Cu is reduced from +2 to zero.
• In other words:
Gaining electrons is reduction. So this is a reduction reaction
• The newly formed copper atoms stick to the surface of the electrode.
• Also, the number of Cu+2 ions present in the solution will go on decreasing. So the blue colour of the solution (which is due to the presence of Cu+2 ions) will fade
• We have seen earlier (when we discussed galvanic cells) that, the electrode at which reduction takes place is the cathode.
• So in the present experiment, the negative electrode is the cathode.
[Recall that, in the galvanic cell, the negative electrode was the anode. Because there, oxidation takes place at the negative electrode].
■ It is interesting to note the following:
• In the present experiment: Negative electrode ⟶ reduction takes place ⟶ cathode
• In galvanic cell: Negative electrode ⟶ oxidation takes place ⟶ anode
This is shown in the fig.12.12 below:
■ Note:
• An electrode may be positive or negative
♦ If oxidation takes place at that electrode, it is the anode
♦ If reduction takes place at that electrode, it is the cathode
4. Now let us continue our discussion:
• The negatively charged Cl-1 ions in the solution move towards the positive electrode. See fig.12.11 above.
• They donate their electrons to that electrode. Thus the circuit is completed. Let us write the equation:
2Cl-1 (aq) ⟶ 2Cl 0 (s) + 2e-1
• This is a oxidation reaction. Because the oxidation number of Cl increases from -1 to zero.
In other words:
Losing electrons is oxidation. So this is an oxidation reaction.
• So chlorine is formed at this electrode. They come out as bubbles from near this electrode.
• We have seen that, the electrode at which oxidation takes place is the anode.
• So in the present experiment, the positive electrode is the anode. [Recall that, in the galvanic cell, the positive electrode was the cathode. Because there, reduction takes place at the positive electrode].
■ It is interesting to note the following:
• In the present experiment: Positive electrode ⟶ oxidation takes place ⟶ anode
• In galvanic cell: Positive electrode ⟶ reduction takes place ⟶ cathode
This is shown in fig.12.13 below:
5. From (3) and (4) we can say that:
■ Both in the present reaction and in the previous galvanic cell,
• The electrode at which oxidation takes place is the anode
• The electrode at which reduction takes place is the cathode
■ But positive and negative electrodes are interchanged:
• In the present reaction, anode is the positive electrode and cathode is the negative electrode
• In the previous galvanic cell, anode is the negative electrode and cathode is the positive electrode
• So we saw how the circuit is completed and current is allowed to pass, when the solid is made into an aqueous solution.
• The passage of current was possible because ions acquired mobility in the aqueous state. ♦ In solid state, ions are held firmly in position. They cannot move.
• This information tells us about another possibility:
If the cupric chloride is heated to a molten state, then also, the ions will acquire mobility. So the circuit will be completed.
• That means, instead of aqueous solution, we can use the molten state of the salt also, for passing electricity.
■ Electrolytes are substances which conduct electricity in molten states or in aqueous solutions and undergo a chemical change. Acids, alkalies and salts are electrolytes in their molten state or in aqueous solution.
■ The process of chemical change taking place in an electrolyte by passing electricity is called electrolysis
■ So now we can define a new type of cell: The electrolytic cell:
In an electrolytic cell, external electricity is allowed to pass through an electrolyte through two electrodes, as a result of which, a chemical reaction takes place in the electrolyte.
• In other words, an electrolytic cell is an apparatus used to carry out electrolysis
The following table 12.2 shows the difference between Galvanic cell and Electrolytic cell
| GALVANIC CELL | ELECTROLYTIC CELL | Remarks | |
|---|---|---|---|
| 1 | Redox reaction produces electricity | Electricity produces redox reaction | |
| 2 | Oxidation takes place at anode | Oxidation takes place at anode | Whatever be the type of cell, anode is where oxidation takes place |
| 3 | Reduction takes place at cathode | Reduction takes place at cathode | Whatever be the type of cell, cathode is where reduction takes place |
| 4 | Anode is the negative electrode | Anode is the positive electrode | |
| 5 | Cathode is the positive electrode | Cathode is the negative electrode |
In the next section, we will see electrolysis of water.