In the previous section, we saw ionic bond and the electron dot diagram. In this section, we will see some solved examples. We will also learn about Cations and Anions
Solved example 3.1
Some elements (symbols are not real) with their atomic number are given below:
9P, 17Q, 10R, 12S
(i) Write the electronic configuration of each element
(ii) Which element is the most stable one? Why?
(iii) Which element donates electrons in chemical reaction?
(iv) Write the chemical formula of the compound formed by combining element S with element P
Solution:
(i) Electronic configuration of the elements are given below (details here):
9P : 2,7 ; 17Q : 2, 8, 7 ; 10R : 2, 8 ; 12S : 2, 8, 2
(ii) 10R is the most stable element. Because, it has 8 electrons in it's outer most shell
(iii) • 9P will have to donate 7 electrons to attain octet. It is easier to accept 1 electron, than to donate 7
• 17Q will have to donate 7 electrons to attain octet. It is easier to accept 1 electron, than to donate 7
• 10R has already has octet. It will not accept or donate any electrons
• 12S can donate 2 electrons to attain octet. It is easier to donate 2, than to accept 6
• So the answer is 12S
(iv) Combination of S and P:
• 12S has a configuration 2, 8, 2. So it has 2 electrons in the outer most shell
• It needs 6 more electrons to attain octet. It is easier to lose the 2 electrons than to obtain 6 electrons
• 9P has a configuration 2, 7. So it has 7 electrons in the outer most shell
• It needs 1 more electron to attain octet.
• So 2 atoms of P will combine with one atom of S. So that:
♦ One P will accept 'one of the 2 electrons' donated by S
♦ The other P will accept the 'other of the 2 electrons' donated by S
• With the above information, we can draw the electron dot diagram as shown in fig.3.7 below:
 |
| Fig.3.7 |
• The outer most electrons are shown as dots around the symbol of the atom
• By counting the number of dots, we can see that, S has lost two electrons, and the two P atoms have gained those two electrons. (one electron for each P)
• The initial and final configurations are also given. S and P have attained octet
• S has become a positive ion. This is due to the loss of electrons. Two electrons are lost. So the charge is 2+. So we write '2+' on the top right
• Each P has become a negative ion. This is due to the gain of electron. One electrons is gained by each P. So the charge is 1- for each. So we write '-' on the top right
• The 'oppositely charged' ions are held together by electrostatic force of attraction. Thus a molecule SP2 is formed
Solved example 3.2
Draw the electron dot diagram for the ionic bonding in the following compounds:
(i) Sodium fluoride (ii) Magnesium fluoride
(Hint: Atomic numbers are: 11Na, 9F, 12Mg)
Solution:
(i) Formation of Sodium fluoride:
• 11Na has a configuration 2, 8, 1. So it has 1 electron in the outer most shell
• It needs 7 more electrons to attain octet. It is easier to lose the 1 electron than to obtain 7 electrons
• 9F has a configuration 2, 7. So it has 7 electrons in the outer most shell
• It needs 1 more electron to attain octet.
• So 1 atom of Na will combine with one atom of F. So that, F will accept the 1 electron donated by Na
• With the above information, we can draw the electron dot diagram as shown in fig.3.8 below:
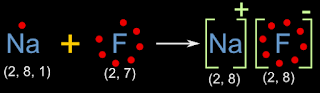 |
| Fig.3.8 |
• The outer most electrons are shown as dots around the symbol of the atom
• By counting the number of dots, we can see that, Na has lost one electrons, and F have gained that electron
• The initial and final configurations are also given. Na and F have attained octet
• Na has become a positive ion. This is due to the loss of electron. One electron is lost. So the charge is 1+. So we write '+' on the top right
• F has become a negative ion. This is due to the gain of electron. One electrons is gained by P. So the charge is 1-. So we write '-' on the top right
• The 'oppositely charged' ions are held together by electrostatic force of attraction. Thus a molecule NaF is formed
(i) Formation of Magnesium fluoride:
• 12Mg has a configuration 2, 8, 2. So it has 2 electrons in the outer most shell
• It needs 6 more electrons to attain octet. It is easier to lose the 2 electrons than to obtain 6 electrons
• 9F has a configuration 2, 7. So it has 7 electrons in the outer most shell
• It needs 1 more electron to attain octet.
• So 2 atoms of F will combine with one atom of Mg. So that:
♦ One F will accept 'one of the 2 electrons' donated by Mg
♦ The other F will accept the 'other of the 2 electrons' donated by Mg
• With the above information, we can draw the electron dot diagram as shown in fig.3.9 below:
 |
| Fig.3.9 |
• The outer most electrons are shown as dots around the symbol of the atom
• By counting the number of dots, we can see that, Mg has lost two electrons, and the two F atoms have gained those two electrons. (one electron for each F)
• The initial and final configurations are also given. Mg and F have attained octet
• Mg has become a positive ion. This is due to the loss of electrons. Two electrons are lost. So the charge is 2+. So we write '2+' on the top right
• Each F has become a negative ion. This is due to the gain of electron. One electrons is gained by each F. So the charge is 1- for each. So we write '-' on the top right
• The 'oppositely charged' ions are held together by electrostatic force of attraction. Thus a molecule MgF2 is formed
Cations and Anions
Let us consider the formation of NaCl that we saw in the previous section. It's electron dot diagram that we saw in fig.3.4 is shown again below:
As a result of the donation and acceptance of electron, 'sodium ion' and 'chlorine ion' is formed. The formation of these ions can be represented using simple equations:
• Na → Na+ + 1e-
• Cl + 1e- → Cl -
• The first equation indicates that: Sodium atom has donated an electron and became a positive ion.
• The second equation indicates that: Chlorine atom has accepted the electron, and became a negative ion.
■ Positive ions are called cations
■ Negative ions are called anions
Another example:
Let us consider the formation of MgO that we saw in fig.3.5 in the previous section. It is shown again below:
The equations can be written as:
• Mg → Mg2+ + 2e-
• O + 2e- → O2-
Here Mg2+ is the cation and O2- is the anion
One more example:
Consider the formation of Na2O that we saw in fig.3.6 in the previous section. It is shown again below:
The equations can be written as:
• 2Na → 2Na1+ + 2e-
• O + 2e- → O2-
Here Na1+ is the cation and O2- is the anion
Now we will see a solved example:
Solved example 3.3
Write all the details regarding the formation of Magnesium chloride MgCl2
Solution:
(i) Formation of Magnesium chloride:
• 12Mg has a configuration 2, 8, 2. So it has 2 electrons in the outer most shell
• It needs 6 more electrons to attain octet. It is easier to lose the 2 electrons than to obtain 6 electrons
• 17Cl has a configuration 2, 8, 7. So it has 7 electrons in the outer most shell
• It needs 1 more electron to attain octet.
• So 2 atoms of Cl will combine with one atom of Mg. So that:
♦ One Cl will accept 'one of the 2 electrons' donated by Mg
♦ The other Cl will accept the 'other of the 2 electrons' donated by Mg
• With the above information, we can draw the electron dot diagram as shown in fig.3.10 below:
 |
| Fig.3.10 |
• The outer most electrons are shown as dots around the symbol of the atom
• By counting the number of dots, we can see that, Mg has lost two electrons, and the two Cl atoms have gained those two electrons. (one electron for each Cl)
• The initial and final configurations are also given. Mg and Cl have attained octet
• Mg has become a positive ion. This is due to the loss of electrons. Two electrons are lost. So the charge is 2+. So we write '2+' on the top right
• Each Cl has become a negative ion. This is due to the gain of electron. One electrons is gained by each Cl. So the charge is 1- for each. So we write '-' on the top right
• The 'oppositely charged' ions are held together by electrostatic force of attraction. Thus a molecule MgCl2 is formed
The ion equations can be written as:
• Mg → Mg2+ + 2e-
• 2Cl + 2e- → 2Cl-
Here Mg2+ is the cation and Cl- is the anion
So we have completed the discussion on Ionic bonds. In the next section, we will see Covalent bonds.
PREVIOUS CONTENTS NEXT
Copyright©2016 High school Chemistry lessons. blogspot.in - All Rights Reserved
In the previous section, we completed the discussion on the Bohr model electronic configuration of atoms. We also saw isotopes. In this section, we will learn about Chemical bonding.
We have seen how the electrons are arranged in the various shells. Each element has a unique arrangement of electrons. This arrangement is called the configuration. We have learned to write the configuration of a large number of elements.
Now we consider the number of electrons in the outer most shell of an atom. Note that, the outer most shell is what matters for our present discussion. We want the number of electrons in this outer most shell.
If the number of electrons in the outer most shell is '8', then it is called an Octet electron configuration. Neon and Argon are good examples. They are shown in the fig. 2.1 below:
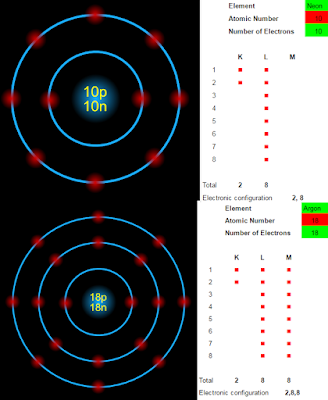 |
| Fig.3.1 |
Elements, whose atoms have octet electron configuration, generally will not take part in chemical reactions. This is because, it is a stable configuration. So what about those elements which do not have this octet?
They will try to attain the octet, so that, there will be stability. Let us see how this octet can be attained:
• We will take the example of Sodium. It has a configuration of 2, 8, 1. (see fig.3.2 below)
• The outer most shell has 1 electron. So it needs 7 more electrons to attain octet
• Another example is Magnesium. It has a configuration of 2, 8, 2. (see fig.3.2 below)
• The outer most shell has 2 electrons. So it needs 6 more electrons to attain octet
• Yet another example is Chlorine. It has a configuration of 2, 8, 7. (see fig.3.2 below)
• The outer most shell has 7 electrons. So it needs 1 more electron to attain octet
There are many such elements which are in 'need of electrons' to attain octet. How do they get the required number of electrons?
■ Consider the example of sodium and chlorine.
• We have seen the configuration of sodium above
• It has 1 electron in the outer most shell. So it needs 7 more electrons to attain octet
• It is easier to 'lose the 1 electron', than to 'obtain 7 electrons'
• When the one electron in the M-shell is taken away, the M-shell as a whole will disappear
• The L-shell will then become the 'outer most shell'
• The L-shell already have 8 electrons. So when L-shell becomes the outer most shell, it is an octet
• Now consider chlorine. We have seen it's configuration above
• It has 7 electrons in it's outer most shell. So it needs one more electron to attain octet
[Another way to attain octet is to lose the 7 electrons in the outer most shell. But it is easier to accept one electron than to lose 7 electrons]
• So it will accept one electron from the sodium atom that we saw above
When this donation and acceptance takes place, some other important changes also occurs:
• Normally, an atom is neutral because, the number of protons is equal to the number of electrons
• But when one electron is donated, the number of protons become excess. It becomes excess by '1'
• So the sodium atom which was initially neutral, has now become 'charged'
• We call such charged particles as ions. When an atom has become charged, we can no longer call it an 'atom'. We call it an 'ion'
• So our 'sodium atom' has become a 'sodium ion'. And this sodium ion has a positive charge
• Similarly, the chlorine atom has accepted an electron
• Number of electrons have become excess by 1
• It has now become a chlorine ion. And this chlorine ion has a negative charge
• So we see that the atoms have become ions
• Sodium atom has become sodium ion, and chlorine atom has become chlorine ion
• An electrostatic force of attraction will develop between the two ions. This force develops because, they are 'oppositely charged'
• This force will hold the two ions together
• Thus a molecule consisting of one ion of sodium and one ion of chlorine will be formed
■ This molecule is the 'molecule of sodium chloride'. It is represented as NaCl
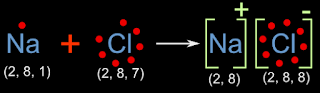
The transfer of electron between sodium and chlorine can be represented pictorially as shown in the fig. 3.3 below:
 |
| Fig.3.3 |
• Consider the left side of the arrow: On either sides of the '+' sign, we have bohr models of sodium and chlorine. This shows the initial state
• Sodium has one electron in the outer most shell. On the right side of the arrow, this atom of sodium has become an 'ion'. The outer most electron is lost. So the bohr model is shown inside square brackets, and a '+' sign is given at top right. This indicates one positive charge
• On the left side of the arrow, the bohr model of the chlorine atom has 7 electrons in the outer most shell. On the right side, the number of electrons in the outer most shell becomes 8. The chlorine atom becomes a chlorine ion. The model is put inside square brackets, and a '-' sign is given at top right. This indicates one negative charge
• Each entity inside square brackets is an ion. These 'oppositely charged' ions are held together by electrostatic force of attraction. So the diagram gives us a clear picture of how sodium and chlorine attain octet. It also shows us the formation of sodium chloride
Another method to represent the above process, is by using Electron dot diagram. In this method, only those electrons in the outer most shell are shown. This is because, they are the only electrons taking part in the bond formation. The dot diagram showing the formation of sodium chloride is shown in fig.3.4 below:
 |
| Fig.3.4 |
• The outer most electrons are shown as dots around the symbol of the atom
• By counting the number of dots, we can see that, sodium has lost one electron, and chlorine has gained one electron
• The initial and final configurations are also given. Sodium and chlorine has attained octet
• Sodium has become a positive ion. This is due to the loss of electron. One electron is lost. so the charge is 1+. For a charge of magnitude one, the '1' is not written. So we just write '+' on the top right
• Chlorine has become a negative ion. This is due to the gain of electron. One electron is gained. so the charge is 1-. For a charge of magnitude one, the '1' is not written. So we just write '-' on the top right
• The 'oppositely charged' ions are held together by electrostatic force of attraction. Thus a molecule NaCl of sodium chloride is formed
Formation of Magnesium oxide
• Magnesium has a configuration 2, 8, 2. (See here). So it has 2 electrons in the outer most shell
• It needs 6 more electrons to attain octet. It is easier to lose the 2 electrons than to obtain 6 electrons
• Oxygen has a configuration 2, 6. So it has 6 electrons in the outer most shell
• It needs 2 more electrons to attain octet. So it will accept the 2 electrons which are donated by magnesium
• With the above information, we can draw the electron dot diagram as shown in fig.3.5 below:
 |
| Fig.3.5 |
• The outer most electrons are shown as dots around the symbol of the atom
• By counting the number of dots, we can see that, magnesium has lost two electrons, and oxygen has gained those two electrons
• The initial and final configurations are also given. Magnesium and oxygen has attained octet
• Magnesium has become a positive ion. This is due to the loss of electrons. Two electrons are lost. So the charge is 2+. So we write '2+' on the top right
• Chlorine has become a negative ion. This is due to the gain of electron. Two electrons are gained. So the charge is 2-. So we write '2-' on the top right
• The 'oppositely charged' ions are held together by electrostatic force of attraction. Thus a molecule MgO of Magnesium oxide is formed
Formation of sodium oxide
• Na has a configuration 2, 8, 1. So it has 1 electron in the outer most shell
• It needs 7 more electrons to attain octet. It is easier to lose the 1 electron than to obtain 7 electrons
• O has a configuration 2, 6. So it has 6 electrons in the outer most shell
• It needs 2 more electron to attain octet.
• So 2 atoms of Na will combine with one atom of O. So that:
♦ One Na will donate one electron to O
♦ The other Na will also donate one electron to O
♦ So, in total, O gets two electrons
• With the above information, we can draw the electron dot diagram as shown in fig.3.6 below:
 |
| Fig.3.6 |
• The outer most electrons are shown as dots around the symbol of the atom
• By counting the number of dots, we can see that, each Na has lost one electron, and the O atom have gained those two electrons. (one electron from each Na)
• The initial and final configurations are also given. Na and O have attained octet
• Na has become a positive ion. This is due to the loss of electron. One electron is lost. So the charge is 1+. So we write '+' on the top right
• O has become a negative ion. This is due to the gain of electron. Two electrons are gained by O. So the charge is 2- . So we write '-' on the top right
• The 'oppositely charged' ions are held together by electrostatic force of attraction. Thus a molecule Na2O is formed
In the next section, we will see some solved examples. We will also learn about Cations and Anions.
PREVIOUS CONTENTS NEXT
Copyright©2016 High school Chemistry lessons. blogspot.in - All Rights Reserved
In the previous section, we saw the Bohr model electronic configuration of atoms. We also saw some solved examples. In this section, we will learn about Isotopes, Isobars and Isotones.
We have seen that the number of protons in an atom is unique. It is the atomic number Z, and it will not change. It is the identity of the element. We have also seen that, in addition to protons, the nucleus contains neutrons also. The number of neutrons may not be the same in all atoms of the same element. Consider an example:
 |
| Fig.2.15 |
• In fig.2.15(a), the atom has 1 proton, 1 electron, and 0 neutron. So it's mass number A = Z + No. Of neutrons = 1 + 0 = 1
• In fig(b), the atom has 1 proton, 1 electron, and 1 neutron. So it's mass number A = Z + No. Of neutrons = 1 + 1 = 2
• In fig(c), the atom has 1 proton, 1 electron, and 2 neutrons. So it's mass number A = Z + No. Of neutrons = 1 + 2 = 3
In all the three cases, the number of protons is the same. So the three atoms belong to the same element. Though they belong to the same element, the mass is different. So we need a method to distinguish between the three. For this, we use the term 'isotopes'. In the above fig.2.15, the three atoms are 'isotopes of the same element'.
In the fig., the element shown is hydrogen. Because Z = 1. So we say: The three atoms are 'isotopes of hydrogen'. The three isotopes of hydrogen are:
• Protium Z = 1, A = 1
• Deutirium Z =1, A = 2
• Tritium Z = 1, A = 3
Representation of Isotopes
We have seen that the elements are represented by their symbols. We have also seen that the Z is written on left side bottom, and A on left side top. This same method can be used to represent isotopes also. The isotopes of hydrogen are represented as shown below:
(a) represents Protium, (b) represents Deuterium, and (c) represents Tritium
In the same way, the isotopes of carbon can be represented as:
(a) represents Carbon-12, (b) represents Carbon-13, and (c) represents Carbon-14
Uses of isotopes
Isotopes find application in many fields. Some examples are:
• Carbon-14 is used to determine the age of fossils and prehistoric objects
• Deuterium is used in atomic reactors
So we have seen the details about isotopes. It involves the combination of two items: (i) Atomic number Z (same), and (ii) Mass number A (different)
Other combinations are also possible. Consider the example given below:
(a) is Argon. It has Z = 18 and A = 40
(b) is Calcium. It has Z = 20 and A = 40
Note that both have A same. Such elements having same mass number but different atomic numbers are called isobars. The combination here is: (i) Atomic number Z (different), and (ii) Mass number A (same).
Another combination that is possible is, (i) Atomic number (different) and (ii) Number of neutrons (same). An example is given below:
(a) is Nitrogen. It has Z = 7 and A = 15. There fore number of neutrons = 15 -7 = 8
(b) is Carbon-14. It has Z = 6 and A = 14. There fore number of neutrons = 14 - 6 = 8
Number of neutrons is the same. Such elements having different atomic numbers but same number of neutrons are called isotones.
We will now see some solved examples
Solved example 2.6
Bohr models of atoms A, B, C and D (symbols are not real) are given below:
(a) Write the atomic number, mass number and electronic configuration of the atoms
(b) Among these, which are isotopes? why?
Solution:
■ (A) has 6 protons in it's nucleus. So the atomic number Z = 6
• It has 6 neutrons in the nucleus. So mass number = No. of protons + No. of neutrons = 6 + 6 = 12
• It has 2 electrons in the K-shell and 4 electrons in the L-shell. So the electronic configuration is 2,4
■ (B) has 7 protons in it's nucleus. So the atomic number Z = 7
• It has 8 neutrons in the nucleus. So mass number = No. of protons + No. of neutrons = 7 + 8 = 15
• It has 2 electrons in the K-shell and 5 electrons in the L-shell. So the electronic configuration is 2,5
■ (C) has 6 protons in it's nucleus. So the atomic number Z = 6
• It has 8 neutrons in the nucleus. So mass number = No. of protons + No. of neutrons = 6 + 8 = 14
• It has 2 electrons in the K-shell and 4 electrons in the L-shell. So the electronic configuration is 2,4
■ (D) has 8 protons in it's nucleus. So the atomic number Z = 8
• It has 8 neutrons in the nucleus. So mass number = No. of protons + No. of neutrons = 6 + 8 = 14
• It has 2 electrons in the K-shell and 6 electrons in the L-shell. So the electronic configuration is 2,6
■ Among the above four atoms, (A) and (C) are isotopes because they both have the same atomic number Z = 6, and different mass numbers. (A) has mass number 12, and (C) has mass number 14. It can be represented as:
Solved example 2.7
Symbols (not real symbols) of some atoms are given below
(a) Find the atomic number and mass number of these elements
(b) Which among these are isotopic pairs
(c) Draw the bohr model of atom Q
Solution:
a. (P) has atomic number Z = 8 and mass number A = 17
(Q) has atomic number Z = 18 and mass number A = 36
(R) has atomic number Z = 8 and mass number A = 16
b. (P) and (R) are isotopic pairs. because they have the same atomic number Z = 8, and different mass numbers. P has mass number = 17 and Q has mass number = 16
c.The Bohr model of atom Q is shown below:
In the next chapter we will see 'Chemical bonding'.
PREVIOUS CONTENTS NEXT
Copyright©2016 High school Chemistry lessons. blogspot.in - All Rights Reserved
In the previous section, we saw the Bohr model electronic configuration of atoms. In this section, we will see some solved examples.
Solved example 2.3
Write the electronic configuration of the following elements and draw their Bohr model
(i) N with Z = 7 and A = 14 (ii) Mg with Z = 12 and A = 24 (iii) S with Z = 16 and A = 32
Solution:
(i) • Given Z = 7 and A = 14.
• So number of protons = number of electrons = 7
• Number of neutrons = A - Z = 14 - 7 = 7. (Number of neutrons must be marked in the nucleus when we draw the Bohr model)
• The electronic configuration can be determined by putting the arrangement of the 7 electrons in a tabular form as shown in fig. 2.10 below:
 |
| Fig.2.10 |
• From the table we can see that, the first 2 electrons fill up the K-shell, and the remaining 5 electrons take positions in the L-shell. So we get K = 2 and L = 5. So the electronic configuration is: 2,5
• Once we get the configuration, the Bohr model can be easily drawn. In the fig.2.10 above, the inner circle (K-shell) has 2 electrons, and the outer circle (L-shell) has 5 electrons
(ii) • Given Z = 12 and A = 24
• So number of protons = number of electrons = 12
• Number of neutrons = A - Z = 24 - 12 = 12. (Number of neutrons must be marked in the nucleus when we draw the Bohr model)
• The electronic configuration can be determined by putting the arrangement of the 12 electrons in a tabular form as shown in fig. 2.11 below:
 |
| Fig.2.11 |
• From the table we can see that, the first 2 electrons fill up the K-shell, next 8 electrons will fill up the L-shell. Maximum no. of electrons that L-shell can hold is 8. So the remaining 2 electrons take positions in the M-shell. So we get K = 2, L = 8 and M = 2. So the electronic configuration is: 2,8,2
• Once we get the configuration, the Bohr model can be easily drawn. In the fig.2.11 above, the inner circle (K-shell) has 2 electrons, the next outer circle (L-shell) has 8 electrons, and the outer most circle (M-shell) has 2 electrons.
(iii) • Given Z = 16 and A = 32
• So number of protons = number of electrons = 16
• Number of neutrons = A - Z = 32 - 16 = 16. (Number of neutrons must be marked in the nucleus when we draw the Bohr model)
• The electronic configuration can be determined by putting the arrangement of the 16 electrons in a tabular form as shown in fig. 2.12 below:
 |
| Fig.2.12 |
• From the table we can see that, the first 2 electrons fill up the K-shell, next 8 electrons will fill up the L-shell. Maximum no. of electrons that L-shell can hold is 8. So the remaining 6 electrons take positions in the M-shell. We get K = 2, L = 8 and M = 6. So the electronic configuration is: 2,8,6
• Once we get the configuration, the Bohr model can be easily drawn. In the fig.2.12 above, the inner circle (K-shell) has 2 electrons, the next outer circle (L-shell) has 8 electrons, and the outer most circle (M-shell) has 6 electrons.
Solved example 2.4
Bohr model of Al (Aluminium) is shown in fig.2.13 below. Analyse it and answer the following questions:
 |
| Fig.2.13 |
(i) Write the Atomic number and mass number of aluminium
(ii) Write the number of protons, neutrons and electrons in an aluminium atom
(iii) Write the electronic configuration of aluminium
Solution:
(i) In the Bohr model, it is written 13p. So the number of protons = 13. Also it is written 14n. So the number of neutrons = 14. Thus we get:
• Atomic number Z = No. of protons = 13
• Mass number A = No. of protons + No. of neutrons = 13 + 14 = 27
(ii) • Number of protons = 13. • No. of neutrons = 14
• No. of electrons in the k-shell = 2, No. of electrons in the L-shell = 8, and the no. of electrons in the M-shell = 3. Thus total no. of electrons = 2 + 8 + 3 = 13
(iii) We wrote the number of electrons in each shell in the previous step. From that, we get electronic configuration = 2,8,3
Solved example 2.5
The mass number of an atom = 31. It has 5 electrons in the M-shell.
(i) Write the electronic configuration of this atom
(ii) What is the atomic number of this atom ?
(iii) How many neutrons does this atom have?
(iv) Draw the Bohr model of this atom
Solution:
(i) Given that the M-shell contains 5 electrons. This is shown in fig.2.14(a) below:
 |
| Fig.2.14 |
• The M-shell can contain upto 2 × 32 = 18 electrons
• If the M-shell is the outermost shell, it can contain upto 8 electrons
• In our case it contains only 5 electrons.
• So we can conclude that, in the given atom, the M-shell is the outermost shell. Because, if it were an inner shell, it would have contained more than 5 electrons
• Two other conclusions can also be made:
♦ The inner K-shell is completely filled up
♦ The inner L-shell is also completely filled up
• The above conclusions are made on the fact that, occupation of M can begin only when K and L are completely filled up. So we get: 2 electrons in K, 8 electrons in L and 5 electrons in M
• Thus the electronic configuration is 2,8,5
(ii) The total number of electrons = 2 + 8 + 5 = 15. So the number of protons is also equal to 15. Thus atomic number Z = 15
(iii) Mass number A is given as 31. So number of neutrons = A - Z = 31 - 15 = 16
(iv) The Bohr model of the given atom is shown in fig.2.14(b) above
In the next section we will see 'Isotopes'.
PREVIOUS CONTENTS NEXT
Copyright©2016 High school Chemistry lessons. blogspot.in - All Rights Reserved
In the previous section, we saw the mass of protons, neutrons and electrons. In this section, we will learn about Mass number and Atomic number. Also later in this section, we will learn about the arrangement of electrons in the atoms.
We have seen that the mass of a proton = 1u, mass of a neutron = 1u, and mass of an electron = 0. Consider an atom in which there are 2 protons, 2 neutrons and 2 electrons. We can calculate the mass of that atom as follows:
• Mass of 2 protons = 2 × 1 u = 2 u
• Mass of 2 neutrons = 2 × 1 u = 2 u
• Mass of 2 electrons = 2 × 0 = 0
∴ Total mass = 2 u + 2 u + 0 = 4 u
The same steps can be written in a shorter form:
• Total mass = 2 u + 2 u + 0
• ⇒ Total mass = (2 + 2) × u = 4 u
What we have inside the parenthesis is the total number of protons and neutrons. So, to calculate the mass of the atom, all we have to do, is to add the number of protons and neutrons, and multiply the sum by u.
The steps become that easy due to the following reasons:
• Both protons and neutrons have the same '1u' as mass. So it can be taken outside the paranthesis
• The mass of electrons do not come into the calculations because it is zero
Let us verify this by taking another example:
An atom has 12 protons, 13 neutrons and 12 electrons. Find the mass of the atom:
Solution:
• Total mass = 12 × 1 u + 13 × 1 u + 12 × 0
• same as Total mass = (12 u + 13 u + 0)
• Same as Total mass = (12 + 13) × u
■ We get the same final step: Add the number of protons and neutrons, and multiply the sum by u.
This can be further simplified:
• If we get the sum of the number of protons and number of neutrons, we can straight away calculate the mass of the atom
• So this sum has special place in chemistry. Because, this sum gives the mass of the atom
• Once we get the sum, all we have to do is to multiply it by 'u'.
As the sum gives the mass directly, it is called the Mass number. So:
■ No. of protons + No. of neutrons = Mass number
■ Mass number is denoted by the letter 'A'
Significance of particles
We have seen the three particles: Protons, neutrons and electrons. Protons and neutrons are inside the nucleus. While electrons revolve around the nucleus in fixed orbits. When the atoms collide with each other, one or more electrons, especially, those in the outer orbits may be knocked out. When this happens, the number of electrons will become less than the number of protons. The ‘equality in the number of protons and electrons’ can change under other conditions also. For example, during chemical reactions, some electrons may get exchanged between atoms. That is., some atoms may lose some electrons, and those electrons will be accepted by some other atoms. So the losing atom will have a decreased number of electrons, while the accepting atom will have an increased number of electrons.
So we see that the number of electrons can change. But such changes do not happen to the number of protons. Because, they are inside the nucleus. So we choose the number protons to identify an element.
■ The total number of protons in an atom is called the Atomic number of that atom. It is represented by the letter Z
Based on A and Z, we may get problems involving the calculations of the ‘number of various particles’. An example is given below:
Solved example 2.1
For an atom, Z = 17, and A = 35. Find the number of protons, neutrons and electrons in that atom.
Solution:
• Given Z = 17 and A = 35
• For any atom, the atomic number Z is the number of protons in it. So we get:
■ No. of protons = 17
• For any atoms (except ions), number of protons is equal to the number of electrons. So we get:
■ No. of electrons = 17
• For any atom, mass number A = No. of protons + No. of neutrons
• So 35 = 17 + no. of neutrons
■ ∴ No. of neutrons = 35 – 17 = 18
Solved example 2.2
The mass of an atom is 4u. It has 2 protons in it's nucleus. How many neutrons does it have?
Solution:
• Given mass of the atom = 4u and No. of protons = 2
• We have, Mass of atom = (No. protons + No. of neutrons) × u
• So we get 4u = (2 + No. of neutrons) × u
• ⇒ 4 = 2 + No. of neutrons
• ∴ No. of neutrons = 4 - 2 = 2
We have learnt about 'symbol of elements'. When we write the symbol, it also represent ‘one atom’ of the element. To this symbol, we can attach the atomic number Z and mass number A. Both are written on the left side. A on left side top and Z on left side bottom. For example, Sodium (Na) has Z = 11 and Z = 23. We can write the symbol as shown in the fig.2.5(a). Fig.2.5(b) shows the general case where X is any element:
 |
| Fig.2.5 |
Arrangement of electrons in an atom
We have seen that the electrons orbit around the nucleus in definite orbits or shells. We have also seen that these orbits are given specific names: K, L, M, N etc., as well as specific numbers: 1, 2, 3, 4 etc.,
The electrons cannot take any orbit they like. There are strict rules by which the electrons are arranged in the orbits. Let us learn those rules:
• Rule 1: The orbits of lower energy levels gets filled first. So the K-shell (No. =1) is filled first. Because it has the lowest energy level. Then the L-shell (No. = 2) is filled. Then M-shell, and so on..
• Rule 2: The maximum number of electrons that can be accommodated in a shell is given by 2n2. Where n is the shell number.
♦ Based on this rule, the maximum number of electrons that can be accommodated in the K-shell = 2 × 12 = 2 × 1 = 2
♦ Maximum number of electrons that can be accommodated in the L-shell = 2 × 22 = 2 × 4 = 8
♦ Maximum number of electrons that can be accommodated in the M-shell = 2 × 32 = 2 × 9 = 18
♦ Maximum number of electrons that can be accommodated in the N-shell = 2 × 42 = 2 × 16 = 32
• Rule 3: The outer most shell in any atom can accommodate upto a maximum of 8 electrons only
We will now arrange the electrons in various atoms. While doing the arrangement, we will see the application of the three rules also. First we take the element hydrogen with atomic number 1. It has only one electron. That electron must occupy the shell with the lowest energy level, which is the K-shell. This is shown in the fig.2.6 below: A tabular form of the arrangement is also shown at the side.
 |
| Fig.2.6 |
In the tabular form, the electron is indicated by a small red square. It comes under the K-shell.
Next consider Helium, which has an atomic number 2. The fig. 2.7 below shows the arrangement:
 |
| Fig.2.7 |
The K-shell can accommodate 2 electrons. So the 2 electrons of Helium will occupy the K-shell.
Next consider Lithium, which has an atomic number 3. The fig. 2.8 below shows the arrangement:
 |
| Fig.2.8 |
The K-shell can accommodate a maximum of only 2 electrons. So the third electron of Lithium must go to the L-shell.
In this way, we can write the configuration for various elements. What happens when the L-shell gets filled up ? The next electron will go to the M-shell. To demonstrate this, we look at the configuration of Neon and Sodium together. The fig. 2.9 below shows the details:
 |
| Fig.2.9 |
Neon has 10 electrons. 2 electrons go to the K-shell. There are 8 electrons remaining. They can occupy the L-shell. But then, the L-shell is filled up. Because 8 is the maximum no. of electrons that the L-shell can accommodate. It does not cause a problem for Neon because, it does not have more than 10 electrons. But when we come to the next element Sodium, the number of electrons is 11. So the eleventh electron must occupy the M-shell.
The configuration of elements up to Argon can be seen here.
In the next section we will see some solved examples.
PREVIOUS CONTENTS NEXT
Copyright©2016 High school Chemistry lessons. blogspot.in - All Rights Reserved



























