In the previous section, we saw ionic bond and the electron dot diagram. In this section, we will see some solved examples. We will also learn about Cations and Anions
Solved example 3.1
Some elements (symbols are not real) with their atomic number are given below:
9P, 17Q, 10R, 12S
(i) Write the electronic configuration of each element
(ii) Which element is the most stable one? Why?
(iii) Which element donates electrons in chemical reaction?
(iv) Write the chemical formula of the compound formed by combining element S with element P
Solution:
(i) Electronic configuration of the elements are given below (details here):
9P : 2,7 ; 17Q : 2, 8, 7 ; 10R : 2, 8 ; 12S : 2, 8, 2
(ii) 10R is the most stable element. Because, it has 8 electrons in it's outer most shell
(iii) • 9P will have to donate 7 electrons to attain octet. It is easier to accept 1 electron, than to donate 7
• 17Q will have to donate 7 electrons to attain octet. It is easier to accept 1 electron, than to donate 7
• 10R has already has octet. It will not accept or donate any electrons
• 12S can donate 2 electrons to attain octet. It is easier to donate 2, than to accept 6
• So the answer is 12S
(iv) Combination of S and P:
• 12S has a configuration 2, 8, 2. So it has 2 electrons in the outer most shell
• It needs 6 more electrons to attain octet. It is easier to lose the 2 electrons than to obtain 6 electrons
• 9P has a configuration 2, 7. So it has 7 electrons in the outer most shell
• It needs 1 more electron to attain octet.
• So 2 atoms of P will combine with one atom of S. So that:
♦ One P will accept 'one of the 2 electrons' donated by S
♦ The other P will accept the 'other of the 2 electrons' donated by S
• With the above information, we can draw the electron dot diagram as shown in fig.3.7 below:
• The outer most electrons are shown as dots around the symbol of the atom
• By counting the number of dots, we can see that, S has lost two electrons, and the two P atoms have gained those two electrons. (one electron for each P)
• The initial and final configurations are also given. S and P have attained octet
• S has become a positive ion. This is due to the loss of electrons. Two electrons are lost. So the charge is 2+. So we write '2+' on the top right
• Each P has become a negative ion. This is due to the gain of electron. One electrons is gained by each P. So the charge is 1- for each. So we write '-' on the top right
• The 'oppositely charged' ions are held together by electrostatic force of attraction. Thus a molecule SP2 is formed
Solved example 3.2
Draw the electron dot diagram for the ionic bonding in the following compounds:
(i) Sodium fluoride (ii) Magnesium fluoride
(Hint: Atomic numbers are: 11Na, 9F, 12Mg)
Solution:
(i) Formation of Sodium fluoride:
• 11Na has a configuration 2, 8, 1. So it has 1 electron in the outer most shell
• It needs 7 more electrons to attain octet. It is easier to lose the 1 electron than to obtain 7 electrons
• 9F has a configuration 2, 7. So it has 7 electrons in the outer most shell
• It needs 1 more electron to attain octet.
• So 1 atom of Na will combine with one atom of F. So that, F will accept the 1 electron donated by Na
• With the above information, we can draw the electron dot diagram as shown in fig.3.8 below:
• The outer most electrons are shown as dots around the symbol of the atom
• By counting the number of dots, we can see that, Na has lost one electrons, and F have gained that electron
• The initial and final configurations are also given. Na and F have attained octet
• Na has become a positive ion. This is due to the loss of electron. One electron is lost. So the charge is 1+. So we write '+' on the top right
• F has become a negative ion. This is due to the gain of electron. One electrons is gained by P. So the charge is 1-. So we write '-' on the top right
• The 'oppositely charged' ions are held together by electrostatic force of attraction. Thus a molecule NaF is formed
(i) Formation of Magnesium fluoride:
• 12Mg has a configuration 2, 8, 2. So it has 2 electrons in the outer most shell
• It needs 6 more electrons to attain octet. It is easier to lose the 2 electrons than to obtain 6 electrons
• 9F has a configuration 2, 7. So it has 7 electrons in the outer most shell
• It needs 1 more electron to attain octet.
• So 2 atoms of F will combine with one atom of Mg. So that:
♦ One F will accept 'one of the 2 electrons' donated by Mg
♦ The other F will accept the 'other of the 2 electrons' donated by Mg
• With the above information, we can draw the electron dot diagram as shown in fig.3.9 below:
• The outer most electrons are shown as dots around the symbol of the atom
• By counting the number of dots, we can see that, Mg has lost two electrons, and the two F atoms have gained those two electrons. (one electron for each F)
• The initial and final configurations are also given. Mg and F have attained octet
• Mg has become a positive ion. This is due to the loss of electrons. Two electrons are lost. So the charge is 2+. So we write '2+' on the top right
• Each F has become a negative ion. This is due to the gain of electron. One electrons is gained by each F. So the charge is 1- for each. So we write '-' on the top right
• The 'oppositely charged' ions are held together by electrostatic force of attraction. Thus a molecule MgF2 is formed
As a result of the donation and acceptance of electron, 'sodium ion' and 'chlorine ion' is formed. The formation of these ions can be represented using simple equations:
• Na → Na+ + 1e-
• Cl + 1e- → Cl -
• The first equation indicates that: Sodium atom has donated an electron and became a positive ion.
• The second equation indicates that: Chlorine atom has accepted the electron, and became a negative ion.
■ Positive ions are called cations
■ Negative ions are called anions
Another example:
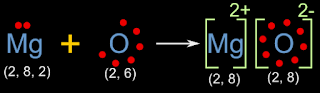
Let us consider the formation of MgO that we saw in fig.3.5 in the previous section. It is shown again below:
The equations can be written as:
• Mg → Mg2+ + 2e-
• O + 2e- → O2-
Here Mg2+ is the cation and O2- is the anion
One more example:
Consider the formation of Na2O that we saw in fig.3.6 in the previous section. It is shown again below:
The equations can be written as:
• 2Na → 2Na1+ + 2e-
• O + 2e- → O2-
Here Na1+ is the cation and O2- is the anion
Now we will see a solved example:
Solved example 3.3
Write all the details regarding the formation of Magnesium chloride MgCl2
Solution:
(i) Formation of Magnesium chloride:
• 12Mg has a configuration 2, 8, 2. So it has 2 electrons in the outer most shell
• It needs 6 more electrons to attain octet. It is easier to lose the 2 electrons than to obtain 6 electrons
• 17Cl has a configuration 2, 8, 7. So it has 7 electrons in the outer most shell
• It needs 1 more electron to attain octet.
• So 2 atoms of Cl will combine with one atom of Mg. So that:
♦ One Cl will accept 'one of the 2 electrons' donated by Mg
♦ The other Cl will accept the 'other of the 2 electrons' donated by Mg
• With the above information, we can draw the electron dot diagram as shown in fig.3.10 below:
• The outer most electrons are shown as dots around the symbol of the atom
• By counting the number of dots, we can see that, Mg has lost two electrons, and the two Cl atoms have gained those two electrons. (one electron for each Cl)
• The initial and final configurations are also given. Mg and Cl have attained octet
• Mg has become a positive ion. This is due to the loss of electrons. Two electrons are lost. So the charge is 2+. So we write '2+' on the top right
• Each Cl has become a negative ion. This is due to the gain of electron. One electrons is gained by each Cl. So the charge is 1- for each. So we write '-' on the top right
• The 'oppositely charged' ions are held together by electrostatic force of attraction. Thus a molecule MgCl2 is formed
The ion equations can be written as:
• Mg → Mg2+ + 2e-
• 2Cl + 2e- → 2Cl-
Here Mg2+ is the cation and Cl- is the anion
So we have completed the discussion on Ionic bonds. In the next section, we will see Covalent bonds.
Solved example 3.1
Some elements (symbols are not real) with their atomic number are given below:
9P, 17Q, 10R, 12S
(i) Write the electronic configuration of each element
(ii) Which element is the most stable one? Why?
(iii) Which element donates electrons in chemical reaction?
(iv) Write the chemical formula of the compound formed by combining element S with element P
Solution:
(i) Electronic configuration of the elements are given below (details here):
9P : 2,7 ; 17Q : 2, 8, 7 ; 10R : 2, 8 ; 12S : 2, 8, 2
(ii) 10R is the most stable element. Because, it has 8 electrons in it's outer most shell
(iii) • 9P will have to donate 7 electrons to attain octet. It is easier to accept 1 electron, than to donate 7
• 17Q will have to donate 7 electrons to attain octet. It is easier to accept 1 electron, than to donate 7
• 10R has already has octet. It will not accept or donate any electrons
• 12S can donate 2 electrons to attain octet. It is easier to donate 2, than to accept 6
• So the answer is 12S
(iv) Combination of S and P:
• 12S has a configuration 2, 8, 2. So it has 2 electrons in the outer most shell
• It needs 6 more electrons to attain octet. It is easier to lose the 2 electrons than to obtain 6 electrons
• 9P has a configuration 2, 7. So it has 7 electrons in the outer most shell
• It needs 1 more electron to attain octet.
• So 2 atoms of P will combine with one atom of S. So that:
♦ One P will accept 'one of the 2 electrons' donated by S
♦ The other P will accept the 'other of the 2 electrons' donated by S
• With the above information, we can draw the electron dot diagram as shown in fig.3.7 below:
 |
| Fig.3.7 |
• By counting the number of dots, we can see that, S has lost two electrons, and the two P atoms have gained those two electrons. (one electron for each P)
• The initial and final configurations are also given. S and P have attained octet
• S has become a positive ion. This is due to the loss of electrons. Two electrons are lost. So the charge is 2+. So we write '2+' on the top right
• Each P has become a negative ion. This is due to the gain of electron. One electrons is gained by each P. So the charge is 1- for each. So we write '-' on the top right
• The 'oppositely charged' ions are held together by electrostatic force of attraction. Thus a molecule SP2 is formed
Solved example 3.2
Draw the electron dot diagram for the ionic bonding in the following compounds:
(i) Sodium fluoride (ii) Magnesium fluoride
(Hint: Atomic numbers are: 11Na, 9F, 12Mg)
Solution:
(i) Formation of Sodium fluoride:
• 11Na has a configuration 2, 8, 1. So it has 1 electron in the outer most shell
• It needs 7 more electrons to attain octet. It is easier to lose the 1 electron than to obtain 7 electrons
• 9F has a configuration 2, 7. So it has 7 electrons in the outer most shell
• It needs 1 more electron to attain octet.
• So 1 atom of Na will combine with one atom of F. So that, F will accept the 1 electron donated by Na
• With the above information, we can draw the electron dot diagram as shown in fig.3.8 below:
 |
| Fig.3.8 |
• By counting the number of dots, we can see that, Na has lost one electrons, and F have gained that electron
• The initial and final configurations are also given. Na and F have attained octet
• Na has become a positive ion. This is due to the loss of electron. One electron is lost. So the charge is 1+. So we write '+' on the top right
• F has become a negative ion. This is due to the gain of electron. One electrons is gained by P. So the charge is 1-. So we write '-' on the top right
• The 'oppositely charged' ions are held together by electrostatic force of attraction. Thus a molecule NaF is formed
(i) Formation of Magnesium fluoride:
• 12Mg has a configuration 2, 8, 2. So it has 2 electrons in the outer most shell
• It needs 6 more electrons to attain octet. It is easier to lose the 2 electrons than to obtain 6 electrons
• 9F has a configuration 2, 7. So it has 7 electrons in the outer most shell
• It needs 1 more electron to attain octet.
• So 2 atoms of F will combine with one atom of Mg. So that:
♦ One F will accept 'one of the 2 electrons' donated by Mg
♦ The other F will accept the 'other of the 2 electrons' donated by Mg
• With the above information, we can draw the electron dot diagram as shown in fig.3.9 below:
 |
| Fig.3.9 |
• By counting the number of dots, we can see that, Mg has lost two electrons, and the two F atoms have gained those two electrons. (one electron for each F)
• The initial and final configurations are also given. Mg and F have attained octet
• Mg has become a positive ion. This is due to the loss of electrons. Two electrons are lost. So the charge is 2+. So we write '2+' on the top right
• Each F has become a negative ion. This is due to the gain of electron. One electrons is gained by each F. So the charge is 1- for each. So we write '-' on the top right
• The 'oppositely charged' ions are held together by electrostatic force of attraction. Thus a molecule MgF2 is formed
Cations and Anions
Let us consider the formation of NaCl that we saw in the previous section. It's electron dot diagram that we saw in fig.3.4 is shown again below:As a result of the donation and acceptance of electron, 'sodium ion' and 'chlorine ion' is formed. The formation of these ions can be represented using simple equations:
• Na → Na+ + 1e-
• Cl + 1e- → Cl -
• The first equation indicates that: Sodium atom has donated an electron and became a positive ion.
• The second equation indicates that: Chlorine atom has accepted the electron, and became a negative ion.
■ Positive ions are called cations
■ Negative ions are called anions
Another example:
Let us consider the formation of MgO that we saw in fig.3.5 in the previous section. It is shown again below:
The equations can be written as:
• Mg → Mg2+ + 2e-
• O + 2e- → O2-
Here Mg2+ is the cation and O2- is the anion
One more example:
Consider the formation of Na2O that we saw in fig.3.6 in the previous section. It is shown again below:
The equations can be written as:
• 2Na → 2Na1+ + 2e-
• O + 2e- → O2-
Here Na1+ is the cation and O2- is the anion
Now we will see a solved example:
Solved example 3.3
Write all the details regarding the formation of Magnesium chloride MgCl2
Solution:
(i) Formation of Magnesium chloride:
• 12Mg has a configuration 2, 8, 2. So it has 2 electrons in the outer most shell
• It needs 6 more electrons to attain octet. It is easier to lose the 2 electrons than to obtain 6 electrons
• 17Cl has a configuration 2, 8, 7. So it has 7 electrons in the outer most shell
• It needs 1 more electron to attain octet.
• So 2 atoms of Cl will combine with one atom of Mg. So that:
♦ One Cl will accept 'one of the 2 electrons' donated by Mg
♦ The other Cl will accept the 'other of the 2 electrons' donated by Mg
• With the above information, we can draw the electron dot diagram as shown in fig.3.10 below:
 |
| Fig.3.10 |
• By counting the number of dots, we can see that, Mg has lost two electrons, and the two Cl atoms have gained those two electrons. (one electron for each Cl)
• The initial and final configurations are also given. Mg and Cl have attained octet
• Mg has become a positive ion. This is due to the loss of electrons. Two electrons are lost. So the charge is 2+. So we write '2+' on the top right
• Each Cl has become a negative ion. This is due to the gain of electron. One electrons is gained by each Cl. So the charge is 1- for each. So we write '-' on the top right
• The 'oppositely charged' ions are held together by electrostatic force of attraction. Thus a molecule MgCl2 is formed
The ion equations can be written as:
• Mg → Mg2+ + 2e-
• 2Cl + 2e- → 2Cl-
Here Mg2+ is the cation and Cl- is the anion
So we have completed the discussion on Ionic bonds. In the next section, we will see Covalent bonds.


No comments:
Post a Comment