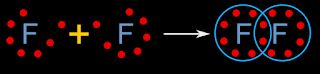
In the previous section, we saw that, the sum of oxidation numbers in any molecule is equal to zero. In this section, we will see a practical application of this property.
It gives the oxidation number of some common elements. Look at the values carefully. Do the values look familiar? Indeed they do. The values are same as the valencies of the corresponding elements. But we must put the appropriate sign ('+' or '-') before the valency values. Let us check for a few elements in the table:
We have seen the details about 'valency calculation' in a previous section here.
■ Consider sodium (Na): It has atomic number = 11. It's electronic configuration is 2,8,1
• The outer most shell has 1 electron. So valency = 1
• Now check the oxidation number corresponding to Na in the table
• It is also 1. With a '+' sign in front. Why this '+' sign?
• Because sodium always donates one electron, and so, it's oxidation number is always positive.
■ Consider aluminium (Al): It has atomic number = 13. It's electronic configuration is 2,8,3
• The outer most shell has 3 electron. So valency = 3
• Now check the oxidation number corresponding to Al in the table
• It is also 3. With a '+' sign in front. Why this '+' sign?
• Because aluminium always donates three electrons, and so, it's oxidation number is always positive.
■ Consider oxygen (O): It has atomic number = 8. It's electronic configuration is 2,6
• The outer most shell has 6 electron. So valency = 8-6 =2
• Now check the oxidation number corresponding to O in the table
• It is also 2. With a '-' sign in front. Why this '-' sign?
• Because oxygen always accepts two electrons, and so, it's oxidation number is always negative.
Once we become familiar with the values in the table, we can think about a more advanced case:
■ What about those elements which are not included in the table? What are their oxidation numbers?
The answer is that, we can find the oxidation number of any component element in a molecule. For that, we have to use two things:
• The oxidation numbers of the common elements given in the table above
• The property that the sum of oxidation numbers in any molecule is equal to zero
With the knowledge of the above two, the procedure is simple, involving basic algebra. Let us see an example:
Find the oxidation number of sulphur in H2SO4
Solution:
• From the table, oxidation number of H = +1
• Oxidation number of O = -2
• Let the oxidation number of S = x
• Sum of oxidation numbers in any molecule is equal to zero. So we can write:
• [2 × (+1)] + [1 × x] + [4 × (-2)] = 0 ⇒ 2 + x - 8 = 0 ⇒ x = 8 - 2 ⇒ x = +6
• So the oxidation number of sulphur in H2SO4 is +6
Now we will see some solved examples:
Solved example 3.7
Find the oxidation number of Manganese (Mn) in (i) KMnO4 , (ii) MnO2 , (iii) Mn2O3 , (iv) Mn2O7
Solution: (i) KMnO4 :
• From the table, oxidation number of K = +1
• Oxidation number of O = -2
• Let the oxidation number of Mn = x
• Sum of oxidation numbers in any molecule is equal to zero. So we can write:
• [1 × (+1)] + [1 × x] + [4 × (-2)] = 0 ⇒ 1 + x - 8 = 0 ⇒ x = 8 - 1 ⇒ x = +7
• So the oxidation number of Mn in KMnO4 is +7
(ii) MnO2
• From the table, oxidation number of O = -2
• Let the oxidation number of Mn = x
• Sum of oxidation numbers in any molecule is equal to zero. So we can write:
• [1 × x] + [2 × (-2)] = 0 ⇒ x - 4 = 0 ⇒ x = 4
• So the oxidation number of Mn in MnO2 is +4
(iii) Mn2O3
• From the table, oxidation number of O = -2
• Let the oxidation number of Mn = x
• Sum of oxidation numbers in any molecule is equal to zero. So we can write:
• [2 × x] + [3 × (-2)] = 0 ⇒ 2x - 6 = 0 ⇒ 2x = 6 ⇒ x = 3
• So the oxidation number of Mn in Mn2O3 is +3
(iii3) Mn2O7
• From the table, oxidation number of O = -2
• Let the oxidation number of Mn = x
• Sum of oxidation numbers in any molecule is equal to zero. So we can write:
• [2 × x] + [7 × (-2)] = 0 ⇒ 2x - 14 = 0 ⇒ 2x = 14 ⇒ x = 7
• So the oxidation number of Mn in Mn2O3 is +7
Solved example 3.8
Oxidation number of oxygen is -2. Find the oxidation number of other atoms in the following compounds:
(i) H2O , (ii) H2CO3 , (iii) HNO3 , (iv) H3PO4
Solution:
(i) H2O
• Given oxidation number of O = -2
• Let the oxidation number of H = x
• Sum of oxidation numbers in any molecule is equal to zero. So we can write:
• [2 × x] + [1 × (-2)] = 0 ⇒ 2x - 2 = 0 ⇒ 2x = 2 ⇒ x = 1
• So the oxidation number of H in H2O is +1
When we solve this problem, we get the oxidation number of H as +1. We can use this value for solving the other problems
(ii) H2CO3
• Given oxidation number of O = -2
• Oxidation number of H = +1
• Let the oxidation number of C = x
• Sum of oxidation numbers in any molecule is equal to zero. So we can write:
• [2 × (+1)] + [1 × x] + [3 × (-2)] = 0 ⇒ 2 + x - 6 = 0 ⇒ x = 4
• So the oxidation number of C in H2CO3 is +4
(iii) HNO3
• Given oxidation number of O = -2
• Oxidation number of H = +1
• Let the oxidation number of N = x
• Sum of oxidation numbers in any molecule is equal to zero. So we can write:
• [1 × (+1)] + [1 × x] + [3 × (-2)] = 0 ⇒ 1 + x - 6 = 0 ⇒ x = 5
• So the oxidation number of N in HNO3 is +5
(iv) H3PO4
• Given oxidation number of O = -2
• Oxidation number of H = +1
• Let the oxidation number of P = x
• Sum of oxidation numbers in any molecule is equal to zero. So we can write:
• [3 × (+1)] + [1 × x] + [4 × (-2)] = 0 ⇒ 3 + x - 8 = 0 ⇒ x = 5
• So the oxidation number of P in H3PO4 is +5
We have completed the discussion on oxidation numbers. In the next chapter, we will learn about the Periodic table.
Method of determining the oxidation number
Consider the following table:It gives the oxidation number of some common elements. Look at the values carefully. Do the values look familiar? Indeed they do. The values are same as the valencies of the corresponding elements. But we must put the appropriate sign ('+' or '-') before the valency values. Let us check for a few elements in the table:
We have seen the details about 'valency calculation' in a previous section here.
■ Consider sodium (Na): It has atomic number = 11. It's electronic configuration is 2,8,1
• The outer most shell has 1 electron. So valency = 1
• Now check the oxidation number corresponding to Na in the table
• It is also 1. With a '+' sign in front. Why this '+' sign?
• Because sodium always donates one electron, and so, it's oxidation number is always positive.
■ Consider aluminium (Al): It has atomic number = 13. It's electronic configuration is 2,8,3
• The outer most shell has 3 electron. So valency = 3
• Now check the oxidation number corresponding to Al in the table
• It is also 3. With a '+' sign in front. Why this '+' sign?
• Because aluminium always donates three electrons, and so, it's oxidation number is always positive.
■ Consider oxygen (O): It has atomic number = 8. It's electronic configuration is 2,6
• The outer most shell has 6 electron. So valency = 8-6 =2
• Now check the oxidation number corresponding to O in the table
• It is also 2. With a '-' sign in front. Why this '-' sign?
• Because oxygen always accepts two electrons, and so, it's oxidation number is always negative.
Once we become familiar with the values in the table, we can think about a more advanced case:
■ What about those elements which are not included in the table? What are their oxidation numbers?
The answer is that, we can find the oxidation number of any component element in a molecule. For that, we have to use two things:
• The oxidation numbers of the common elements given in the table above
• The property that the sum of oxidation numbers in any molecule is equal to zero
With the knowledge of the above two, the procedure is simple, involving basic algebra. Let us see an example:
Find the oxidation number of sulphur in H2SO4
Solution:
• From the table, oxidation number of H = +1
• Oxidation number of O = -2
• Let the oxidation number of S = x
• Sum of oxidation numbers in any molecule is equal to zero. So we can write:
• [2 × (+1)] + [1 × x] + [4 × (-2)] = 0 ⇒ 2 + x - 8 = 0 ⇒ x = 8 - 2 ⇒ x = +6
• So the oxidation number of sulphur in H2SO4 is +6
Now we will see some solved examples:
Solved example 3.7
Find the oxidation number of Manganese (Mn) in (i) KMnO4 , (ii) MnO2 , (iii) Mn2O3 , (iv) Mn2O7
Solution: (i) KMnO4 :
• From the table, oxidation number of K = +1
• Oxidation number of O = -2
• Let the oxidation number of Mn = x
• Sum of oxidation numbers in any molecule is equal to zero. So we can write:
• [1 × (+1)] + [1 × x] + [4 × (-2)] = 0 ⇒ 1 + x - 8 = 0 ⇒ x = 8 - 1 ⇒ x = +7
• So the oxidation number of Mn in KMnO4 is +7
(ii) MnO2
• From the table, oxidation number of O = -2
• Let the oxidation number of Mn = x
• Sum of oxidation numbers in any molecule is equal to zero. So we can write:
• [1 × x] + [2 × (-2)] = 0 ⇒ x - 4 = 0 ⇒ x = 4
• So the oxidation number of Mn in MnO2 is +4
(iii) Mn2O3
• From the table, oxidation number of O = -2
• Let the oxidation number of Mn = x
• Sum of oxidation numbers in any molecule is equal to zero. So we can write:
• [2 × x] + [3 × (-2)] = 0 ⇒ 2x - 6 = 0 ⇒ 2x = 6 ⇒ x = 3
• So the oxidation number of Mn in Mn2O3 is +3
(iii3) Mn2O7
• From the table, oxidation number of O = -2
• Let the oxidation number of Mn = x
• Sum of oxidation numbers in any molecule is equal to zero. So we can write:
• [2 × x] + [7 × (-2)] = 0 ⇒ 2x - 14 = 0 ⇒ 2x = 14 ⇒ x = 7
• So the oxidation number of Mn in Mn2O3 is +7
Solved example 3.8
Oxidation number of oxygen is -2. Find the oxidation number of other atoms in the following compounds:
(i) H2O , (ii) H2CO3 , (iii) HNO3 , (iv) H3PO4
Solution:
(i) H2O
• Given oxidation number of O = -2
• Let the oxidation number of H = x
• Sum of oxidation numbers in any molecule is equal to zero. So we can write:
• [2 × x] + [1 × (-2)] = 0 ⇒ 2x - 2 = 0 ⇒ 2x = 2 ⇒ x = 1
• So the oxidation number of H in H2O is +1
When we solve this problem, we get the oxidation number of H as +1. We can use this value for solving the other problems
(ii) H2CO3
• Given oxidation number of O = -2
• Oxidation number of H = +1
• Let the oxidation number of C = x
• Sum of oxidation numbers in any molecule is equal to zero. So we can write:
• [2 × (+1)] + [1 × x] + [3 × (-2)] = 0 ⇒ 2 + x - 6 = 0 ⇒ x = 4
• So the oxidation number of C in H2CO3 is +4
(iii) HNO3
• Given oxidation number of O = -2
• Oxidation number of H = +1
• Let the oxidation number of N = x
• Sum of oxidation numbers in any molecule is equal to zero. So we can write:
• [1 × (+1)] + [1 × x] + [3 × (-2)] = 0 ⇒ 1 + x - 6 = 0 ⇒ x = 5
• So the oxidation number of N in HNO3 is +5
(iv) H3PO4
• Given oxidation number of O = -2
• Oxidation number of H = +1
• Let the oxidation number of P = x
• Sum of oxidation numbers in any molecule is equal to zero. So we can write:
• [3 × (+1)] + [1 × x] + [4 × (-2)] = 0 ⇒ 3 + x - 8 = 0 ⇒ x = 5
• So the oxidation number of P in H3PO4 is +5
We have completed the discussion on oxidation numbers. In the next chapter, we will learn about the Periodic table.