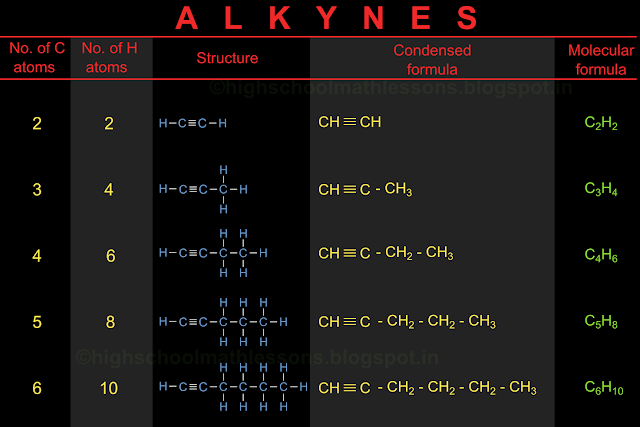
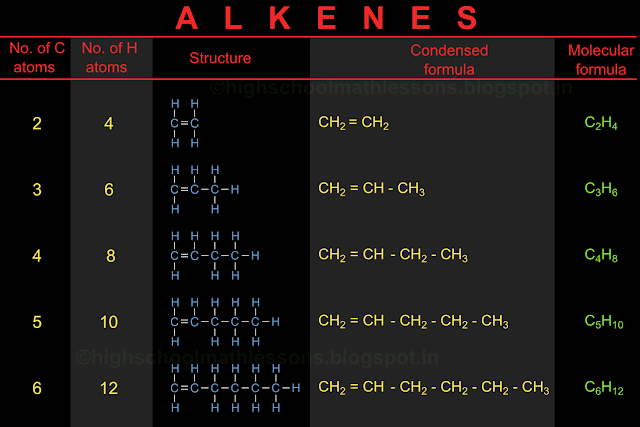
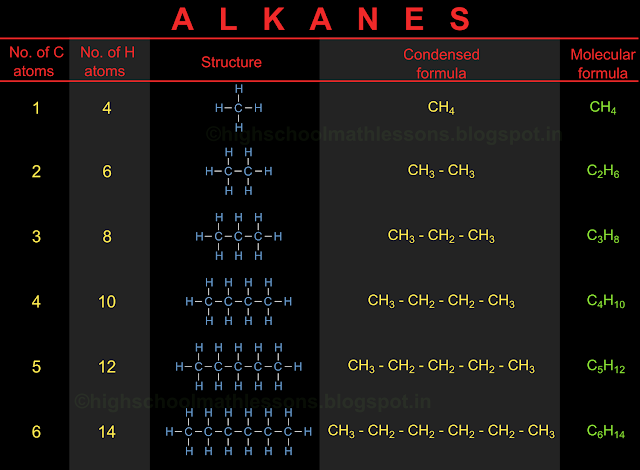
In the previous section, we saw the details and nomenclature of cyclic hydrocarbons. In this section we will learn about the position of various atoms in a hydrocarbon, in 3D space.
We have seen the arrangement of carbon and hydrogen atoms in various hydrocarbons. Consider methane. We have seen the structural formula here. So we know that there are 4 hydrogen atoms around a single carbon atom.
Are the four hydrogen atoms placed in a circle around the carbon atom?
Or are they placed in a square, with one atom at each corner of the square?
Or is it any other shape?
So we have seen how the atoms are arranged in different molecules of hydrocarbons. In the next section, we will learn about Allotropes.
We have seen the arrangement of carbon and hydrogen atoms in various hydrocarbons. Consider methane. We have seen the structural formula here. So we know that there are 4 hydrogen atoms around a single carbon atom.
Are the four hydrogen atoms placed in a circle around the carbon atom?
Or are they placed in a square, with one atom at each corner of the square?
Or is it any other shape?
To find the answer, we must look into the 3D view of a methane molecule.
1. In fig.8.17(a) below, a geometrical solid is shown. It is called a tetrahedron.
Let us see the peculiarities of a tetrahedron in general:
• It is a triangular pyramid. That means, it is a pyramid, whose base is a triangle. The base triangle of our tetrahedron is named as ABC
• It has four faces. One base and three lateral faces.
• All the faces are triangles.
♦ All those triangles are equilateral triangles.
♦ All those equilateral triangles are identical
• It has four corners. In fig.a, they are named as A, B, C and D.
2. The four hydrogen atoms of methane are situated at the four corners of the tetrahedron as shown in fig.8.17(b). They are shown as four pink spheres.
3. The carbon atom is shown as a larger blue sphere. It is situated at the exact centroid of the tetrahedron.
4. The white cylinders indicate the bonds.
5. It is a ball and stick model
6. Upon seeing the arrangement in fig.b, our first impression would be this:
"There is no vertical symmetry"
We feel this because:
• The carbon atom is nearer to the base triangle.
• The distance of the carbon atom from the top corner is greater than it's distance from the base triangle.
7. But the fact is that, the molecule as a whole is symmetrical in any direction. Even if we spin the molecule in any direction, it will be symmetrical. This is because, the distance of the carbon atom from any of the 4 hydrogen atoms is the same.
• All atoms at the base of solid triangles are above the plane of paper
• All atoms at the base of dashed triangles are below the plane of paper
7. Let us see the example of Ethane. It is shown in fig.8.20(a) below
8. Fig.8.20(b) shows some more details.
• All the atoms which fall in the thick red line, lies on the plane of paper.
• The hydrogen atoms with in green circles lies above the plane of paper
• The hydrogen atoms with in yellow circles lies below the plane of paper
1. In fig.8.17(a) below, a geometrical solid is shown. It is called a tetrahedron.
 |
| Fig.8.17 |
Let us see the peculiarities of a tetrahedron in general:
• It is a triangular pyramid. That means, it is a pyramid, whose base is a triangle. The base triangle of our tetrahedron is named as ABC
• It has four faces. One base and three lateral faces.
• All the faces are triangles.
♦ All those triangles are equilateral triangles.
♦ All those equilateral triangles are identical
• It has four corners. In fig.a, they are named as A, B, C and D.
2. The four hydrogen atoms of methane are situated at the four corners of the tetrahedron as shown in fig.8.17(b). They are shown as four pink spheres.
3. The carbon atom is shown as a larger blue sphere. It is situated at the exact centroid of the tetrahedron.
4. The white cylinders indicate the bonds.
5. It is a ball and stick model
6. Upon seeing the arrangement in fig.b, our first impression would be this:
"There is no vertical symmetry"
We feel this because:
• The carbon atom is nearer to the base triangle.
• The distance of the carbon atom from the top corner is greater than it's distance from the base triangle.
7. But the fact is that, the molecule as a whole is symmetrical in any direction. Even if we spin the molecule in any direction, it will be symmetrical. This is because, the distance of the carbon atom from any of the 4 hydrogen atoms is the same.
Now we know the 3 dimensional arrangement of atoms in a molecule of methane. But it is difficult to draw 3D views every time. So we need a method to represent a 3 dimensional arrangement on paper.
1. Consider fig.8.18(a) below. It shows one molecule of methane.
 |
| Fig.8.18 |
2. We want to represent it on paper. The 5 atoms are situated at different directions. There does not seem to be a way to bring them together.
3. But there is good news. On careful analysis, we find that three of them fall in one plane. This plane is shown in fig.8.18(b). The three atoms are:
• The hydrogen atom marked as C
• The hydrogen atom marked as D
• The carbon atom
4. This plane is our 'plane of paper'. The three atoms will fall on the paper.
5. But two hydrogen atoms remain.
• One of them marked as B, projects above the plane of paper, towards the viewer.
• The other marked as A, recedes below the paper, away from the viewer.
6. So we need special methods to represent them. This can be explained with the help of fig.8.19 below.
 |
| Fig.8.19 |
The fig. is based on the following rules:
• All atoms at the ends of single lines fall on the plane of paper• All atoms at the base of solid triangles are above the plane of paper
• All atoms at the base of dashed triangles are below the plane of paper
7. Let us see the example of Ethane. It is shown in fig.8.20(a) below
 |
| Fig.8.20 |
• All the atoms which fall in the thick red line, lies on the plane of paper.
• The hydrogen atoms with in green circles lies above the plane of paper
• The hydrogen atoms with in yellow circles lies below the plane of paper
So we have seen how the atoms are arranged in different molecules of hydrocarbons. In the next section, we will learn about Allotropes.