In the previous section, we saw that 18 grams of water will give water molecules. In this section we will see two more examples like that:
■ Consider a molecule of carbon dioxide (CO2). How many grams of carbon dioxide should we take so that, there will be NA molecules of CO2?
Solution:
Based on the above discussion, we can now take up 'mole'. We will see it in the next section.
■ Consider a molecule of carbon dioxide (CO2). How many grams of carbon dioxide should we take so that, there will be NA molecules of CO2?
Solution:
1. If there is to be NA molecules of CO2, there must be:
• NA carbon atoms and
• (NA × 2 = 2NA) oxygen atoms
2. What is the mass of NA carbon atoms?
• Mass of NA atoms of any element is it's GAM
• So mass of NA carbon atoms is GAM of carbon which is equal to 12 grams
3. What is the mass of NA oxygen atoms?
• Mass of NA atoms of any element is it's GAM
• So mass of NA oxygen atoms is GAM of oxygen which is equal to 16 grams
• So mass of 2NA oxygen atoms = 2 × 16 = 32 grams
4. It seems that 12 grams of carbon and 32 grams of oxygen will give us NA carbon dioxide molecules. Let us check:
5. Let us consider (12+32) = 44 grams of water. Let this '44 grams' consist of 12 grams of carbon and 32 grams of oxygen
6. We can group it in the following way:
• The NA carbon atoms (obtained from 12 grams of carbon) should be split into NA groups, so that, each group has 1 carbon atom
• The 2NA oxygen atoms (obtained from 32 grams of oxygen) should also be split into NA groups, so that, each group has 2 oxygen atoms
7. Thus the 12 grams of carbon is now split into NA groups. Let this be Set 1
• The 32 grams of oxygen is also now split into NA groups. Let this be Set 2
The two sets are shown in fig.10.4 below:
8. Both the sets have the same number (NA) of groups
• So each group from Set 1 will get a partner from Set 2
9. When a group from Set 1 (consisting of one carbon atom) enter into partnership with a group from Set 2 (consisting of two oxygen atoms), we get a molecule (CO2) of carbon dioxide
10. Since there are NA groups in each set, we will get NA carbon dioxide molecules
11. Thus we can conclude: 44 grams of carbon dioxide will give NA carbon dioxide molecules
One more example: Consider a molecule of glucose (C6H12O6). How many grams of glucose should we take so that, there will be NA molecules of C6H12O6?
Solution:
1. If there is to be NA molecules of C6H12O6, there must be:
• (NA × 6 = 6NA) carbon atoms
• (NA × 12 = 12NA) hydrogen atoms
• (NA × 6 = 6NA) oxygen atoms
2. What is the mass of NA carbon atoms?
• Mass of NA atoms of any element is it's GAM
• So mass of NA carbon atoms is GAM of carbon which is equal to 12 grams
• So mass of 6NA carbon atoms = 6 × 12 = 72 grams
3. What is the mass of NA hydrogen atoms?
• Mass of NA atoms of any element is it's GAM
• So mass of NA hydrogen atoms is GAM of oxygen which is equal to 1 gram
• So mass of 12NA oxygen atoms = 12 × 1 = 12 grams
4. What is the mass of NA oxygen atoms?
• Mass of NA atoms of any element is it's GAM
• So mass of NA oxygen atoms is GAM of oxygen which is equal to 16 grams
• So mass of 6NA oxygen atoms = 6 × 16 = 96 grams
5. It seems that 72 grams of carbon, 12 grams of hydrogen and 96 grams of oxygen will give us NA carbon glucose molecules. Let us check:
6. Let us consider (72+12+96) = 180 grams of glucose. Let this '180 grams' consist of 72 grams of carbon, 12 grams of hydrogen and 96 grams of oxygen
7. We can group it in the following way:
• The 6NA carbon atoms (obtained from 72 grams of carbon) should be split into NA groups, so that, each group has 6 carbon atoms
• The 12NA hydrogen atoms (obtained from 12 grams of hydrogen) should also be split into NA groups, so that, each group has 12 hydrogen atoms
• The 6NA oxygen atoms (obtained from 96 grams of oxygen) should also be split into NA groups, so that, each group has 6 oxygen atoms
8. Thus the 72 grams of carbon is now split into NA groups. Let this be Set 1
• The 12 grams of hydrogen is also now split into NA groups. Let this be Set 2
• The 96 grams of oxygen is also now split into NA groups. Let this be Set 3
The three sets are shown in fig.10.5 below:
9. All the three sets have the same number (NA) of groups
• So each group from Set 1 will get a partner from Set 2 and set 3
10. When a group from Set 1 (consisting of six carbon atoms) enter into partnership with a group from Set 2 (consisting of twelve hydrogen atoms) and a group from Set 3 (consisting of six oxygen atoms), we get a molecule (C6H12O6) of glucose
11. Since there are NA groups in each set, we will get NA glucose molecules
12. Thus we can conclude: 180 grams of glucose will give NA glucose molecules
Let us write a summary of the above discussion on molecules:
■ Molecules of elements:
• To get NA hydrogen molecules, we must take 2 grams of hydrogen atoms
• To get NA oxygen molecules, we must take 32 grams of oxygen atoms
• To get NA ozone molecules, we must take 48 grams of oxygen atoms
• To get NA sulphur molecules, we must take 256 grams of sulphur atoms
• To get NA helium molecules, we must take 4 grams of helium atoms
■ Molecules of compounds
• To get NA water molecules, we must take 18 grams of water
• To get NA carbon dioxide molecules, we must take 44 grams of carbon dioxide
• To get NA glucose molecules, we must take 180 grams of glucose
1. The molecule is H2. It has two hydrogen atoms.
2. So total mass of a molecule of hydrogen will be twice the mass of a hydrogen atom
3. We know that, the mass of a hydrogen atom is 1 u
4. So mass of a hydrogen molecule = (2 × 1 u) = 2 u
5. Above we found that, to get NA hydrogen molecules, we must take 2 grams of hydrogen atoms
• In (4) we have 2 u.
• In (5) we have 2 grams
• The numeric parts are the same
■ Consider a molecule of oxygen.
1. The molecule is O2. It has two oxygen atoms.
2. So total mass of a molecule of oxygen will be twice the mass of a oxygen atom
3. We know that, the mass of a oxygen atom is 16 u
4. So mass of a oxygen molecule = (2 × 16 u) = 32 u
5. Above we found that, to get NA oxygen molecules, we must take 32 grams of oxygen atoms
• In (4) we have 32 u.
• In (5) we have 32 grams
• The numeric parts are the same
■ Consider a molecule of ozone.
1. The molecule is O3. It has three oxygen atoms.
2. So total mass of a molecule of ozone will be thrice the mass of a oxygen atom
3. We know that, the mass of a oxygen atom is 16 u
4. So mass of a ozone molecule = (3 × 16 u) = 48 u
5. Above we found that, to get NA ozone molecules, we must take 48 grams of oxygen atoms
• In (4) we have 48 u.
• In (5) we have 48 grams
• The numeric parts are the same
■ Consider a molecule of sulphur.
1. The molecule is S8. It has eight sulphur atoms.
2. So total mass of a molecule of sulphur will be eight times the mass of a sulphur atom
3. We know that, the mass of a sulphur atom is 32 u
4. So mass of a sulphur molecule = (8 × 32 u) = 256 u
5. Above we found that, to get NA sulphur molecules, we must take 256 grams of sulphur atoms
• In (4) we have 256 u.
• In (5) we have 256 grams
• The numeric parts are the same
■ Consider a molecule of helium.
1. The molecule is H. It has only one helium atom.
2. So total mass of a molecule of helium will be same as the mass of a helium atom
3. We know that, the mass of a helium atom is 4 u
4. So mass of a helium molecule = (1 × 4 u) = 4 u
5. Above we found that, to get NA helium molecules, we must take 4 grams of helium atoms
• In (4) we have 4 u.
• In (5) we have 4 grams
• The numeric parts are the same
■ Consider a molecule of water.
1. The molecule is H2O. It has two hydrogen atoms and one oxygen atom.
2. So total mass of a molecule of water will be the sum of:
• twice the mass of a hydrogen atom
• mass of an oxygen atom
3. We know that, the mass of a hydrogen atom is 1 u and that of oxygen atom is 16 u
4. So mass of a water molecule = (2 × 1 u) + (1 × 16 u) = 2 u + 16 u = 18 u
5. Above we found that, to get NA water molecules, we must take 18 grams of water
• In (4) we have 18 u.
• In (5) we have 18 grams
• The numeric parts are the same
■ Consider a molecule of carbon dioxide.
1. The molecule is CO2. It has one carbon atom and two oxygen atoms.
2. So total mass of a molecule of carbon dioxide will be the sum of:
• mass of a carbon atom
• twice the mass of an oxygen atom
3. We know that, the mass of a carbon atom is 12 u and that of oxygen atom is 16 u
4. So mass of a carbon dioxide molecule = (1 × 12 u) + (2 × 16 u) = 12 u + 32 u = 44 u
5. Above we found that, to get NA carbon dioxide molecules, we must take 44 grams of carbon dioxide
• In (4) we have 44 u.
• In (5) we have 44 grams
• The numeric parts are the same
■ Consider a molecule of glucose.
1. The molecule is C6H12O6. It has six carbon atoms, twelve hydrogen atoms and six oxygen atoms.
2. So total mass of a molecule of glucose will be the sum of:
• six times the mass of a carbon atom
• twelve times the mass of a hydrogen atom
• six times the mass of an oxygen atom
3. We know that, the mass of a carbon atom is 12 u, mass of a hydrogen atom is 1 u and that of an oxygen atom is 16 u
4. So mass of a glucose molecule = (6 × 12 u) + (12 × 1 u) + (6 × 16 u) = 72 u + 12 u + 96 u = 180 u
5. Above we found that, to get NA glucose molecules, we must take 180 grams of glucose
• In (4) we have 180 u.
• In (5) we have 180 grams
• The numeric parts are the same
• NA carbon atoms and
• (NA × 2 = 2NA) oxygen atoms
2. What is the mass of NA carbon atoms?
• Mass of NA atoms of any element is it's GAM
• So mass of NA carbon atoms is GAM of carbon which is equal to 12 grams
3. What is the mass of NA oxygen atoms?
• Mass of NA atoms of any element is it's GAM
• So mass of NA oxygen atoms is GAM of oxygen which is equal to 16 grams
• So mass of 2NA oxygen atoms = 2 × 16 = 32 grams
4. It seems that 12 grams of carbon and 32 grams of oxygen will give us NA carbon dioxide molecules. Let us check:
5. Let us consider (12+32) = 44 grams of water. Let this '44 grams' consist of 12 grams of carbon and 32 grams of oxygen
6. We can group it in the following way:
• The NA carbon atoms (obtained from 12 grams of carbon) should be split into NA groups, so that, each group has 1 carbon atom
• The 2NA oxygen atoms (obtained from 32 grams of oxygen) should also be split into NA groups, so that, each group has 2 oxygen atoms
7. Thus the 12 grams of carbon is now split into NA groups. Let this be Set 1
• The 32 grams of oxygen is also now split into NA groups. Let this be Set 2
The two sets are shown in fig.10.4 below:
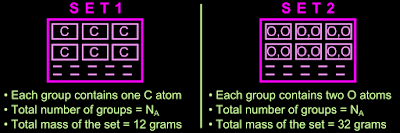 |
| Fig.10.4 |
• So each group from Set 1 will get a partner from Set 2
9. When a group from Set 1 (consisting of one carbon atom) enter into partnership with a group from Set 2 (consisting of two oxygen atoms), we get a molecule (CO2) of carbon dioxide
10. Since there are NA groups in each set, we will get NA carbon dioxide molecules
11. Thus we can conclude: 44 grams of carbon dioxide will give NA carbon dioxide molecules
One more example: Consider a molecule of glucose (C6H12O6). How many grams of glucose should we take so that, there will be NA molecules of C6H12O6?
Solution:
1. If there is to be NA molecules of C6H12O6, there must be:
• (NA × 6 = 6NA) carbon atoms
• (NA × 12 = 12NA) hydrogen atoms
• (NA × 6 = 6NA) oxygen atoms
2. What is the mass of NA carbon atoms?
• Mass of NA atoms of any element is it's GAM
• So mass of NA carbon atoms is GAM of carbon which is equal to 12 grams
• So mass of 6NA carbon atoms = 6 × 12 = 72 grams
3. What is the mass of NA hydrogen atoms?
• Mass of NA atoms of any element is it's GAM
• So mass of NA hydrogen atoms is GAM of oxygen which is equal to 1 gram
• So mass of 12NA oxygen atoms = 12 × 1 = 12 grams
4. What is the mass of NA oxygen atoms?
• Mass of NA atoms of any element is it's GAM
• So mass of NA oxygen atoms is GAM of oxygen which is equal to 16 grams
• So mass of 6NA oxygen atoms = 6 × 16 = 96 grams
5. It seems that 72 grams of carbon, 12 grams of hydrogen and 96 grams of oxygen will give us NA carbon glucose molecules. Let us check:
6. Let us consider (72+12+96) = 180 grams of glucose. Let this '180 grams' consist of 72 grams of carbon, 12 grams of hydrogen and 96 grams of oxygen
7. We can group it in the following way:
• The 6NA carbon atoms (obtained from 72 grams of carbon) should be split into NA groups, so that, each group has 6 carbon atoms
• The 12NA hydrogen atoms (obtained from 12 grams of hydrogen) should also be split into NA groups, so that, each group has 12 hydrogen atoms
• The 6NA oxygen atoms (obtained from 96 grams of oxygen) should also be split into NA groups, so that, each group has 6 oxygen atoms
8. Thus the 72 grams of carbon is now split into NA groups. Let this be Set 1
• The 12 grams of hydrogen is also now split into NA groups. Let this be Set 2
• The 96 grams of oxygen is also now split into NA groups. Let this be Set 3
The three sets are shown in fig.10.5 below:
 |
| Fig.10.5 |
• So each group from Set 1 will get a partner from Set 2 and set 3
10. When a group from Set 1 (consisting of six carbon atoms) enter into partnership with a group from Set 2 (consisting of twelve hydrogen atoms) and a group from Set 3 (consisting of six oxygen atoms), we get a molecule (C6H12O6) of glucose
11. Since there are NA groups in each set, we will get NA glucose molecules
12. Thus we can conclude: 180 grams of glucose will give NA glucose molecules
Let us write a summary of the above discussion on molecules:
■ Molecules of elements:
• To get NA hydrogen molecules, we must take 2 grams of hydrogen atoms
• To get NA oxygen molecules, we must take 32 grams of oxygen atoms
• To get NA ozone molecules, we must take 48 grams of oxygen atoms
• To get NA sulphur molecules, we must take 256 grams of sulphur atoms
• To get NA helium molecules, we must take 4 grams of helium atoms
■ Molecules of compounds
• To get NA water molecules, we must take 18 grams of water
• To get NA carbon dioxide molecules, we must take 44 grams of carbon dioxide
• To get NA glucose molecules, we must take 180 grams of glucose
Is there an easier method to get the above masses? Let us try:
■ Consider a molecule of hydrogen. 1. The molecule is H2. It has two hydrogen atoms.
2. So total mass of a molecule of hydrogen will be twice the mass of a hydrogen atom
3. We know that, the mass of a hydrogen atom is 1 u
4. So mass of a hydrogen molecule = (2 × 1 u) = 2 u
5. Above we found that, to get NA hydrogen molecules, we must take 2 grams of hydrogen atoms
• In (4) we have 2 u.
• In (5) we have 2 grams
• The numeric parts are the same
■ Consider a molecule of oxygen.
1. The molecule is O2. It has two oxygen atoms.
2. So total mass of a molecule of oxygen will be twice the mass of a oxygen atom
3. We know that, the mass of a oxygen atom is 16 u
4. So mass of a oxygen molecule = (2 × 16 u) = 32 u
5. Above we found that, to get NA oxygen molecules, we must take 32 grams of oxygen atoms
• In (4) we have 32 u.
• In (5) we have 32 grams
• The numeric parts are the same
■ Consider a molecule of ozone.
1. The molecule is O3. It has three oxygen atoms.
2. So total mass of a molecule of ozone will be thrice the mass of a oxygen atom
3. We know that, the mass of a oxygen atom is 16 u
4. So mass of a ozone molecule = (3 × 16 u) = 48 u
5. Above we found that, to get NA ozone molecules, we must take 48 grams of oxygen atoms
• In (4) we have 48 u.
• In (5) we have 48 grams
• The numeric parts are the same
■ Consider a molecule of sulphur.
1. The molecule is S8. It has eight sulphur atoms.
2. So total mass of a molecule of sulphur will be eight times the mass of a sulphur atom
3. We know that, the mass of a sulphur atom is 32 u
4. So mass of a sulphur molecule = (8 × 32 u) = 256 u
5. Above we found that, to get NA sulphur molecules, we must take 256 grams of sulphur atoms
• In (4) we have 256 u.
• In (5) we have 256 grams
• The numeric parts are the same
■ Consider a molecule of helium.
1. The molecule is H. It has only one helium atom.
2. So total mass of a molecule of helium will be same as the mass of a helium atom
3. We know that, the mass of a helium atom is 4 u
4. So mass of a helium molecule = (1 × 4 u) = 4 u
5. Above we found that, to get NA helium molecules, we must take 4 grams of helium atoms
• In (4) we have 4 u.
• In (5) we have 4 grams
• The numeric parts are the same
■ Consider a molecule of water.
1. The molecule is H2O. It has two hydrogen atoms and one oxygen atom.
2. So total mass of a molecule of water will be the sum of:
• twice the mass of a hydrogen atom
• mass of an oxygen atom
3. We know that, the mass of a hydrogen atom is 1 u and that of oxygen atom is 16 u
4. So mass of a water molecule = (2 × 1 u) + (1 × 16 u) = 2 u + 16 u = 18 u
5. Above we found that, to get NA water molecules, we must take 18 grams of water
• In (4) we have 18 u.
• In (5) we have 18 grams
• The numeric parts are the same
■ Consider a molecule of carbon dioxide.
1. The molecule is CO2. It has one carbon atom and two oxygen atoms.
2. So total mass of a molecule of carbon dioxide will be the sum of:
• mass of a carbon atom
• twice the mass of an oxygen atom
3. We know that, the mass of a carbon atom is 12 u and that of oxygen atom is 16 u
4. So mass of a carbon dioxide molecule = (1 × 12 u) + (2 × 16 u) = 12 u + 32 u = 44 u
5. Above we found that, to get NA carbon dioxide molecules, we must take 44 grams of carbon dioxide
• In (4) we have 44 u.
• In (5) we have 44 grams
• The numeric parts are the same
■ Consider a molecule of glucose.
1. The molecule is C6H12O6. It has six carbon atoms, twelve hydrogen atoms and six oxygen atoms.
2. So total mass of a molecule of glucose will be the sum of:
• six times the mass of a carbon atom
• twelve times the mass of a hydrogen atom
• six times the mass of an oxygen atom
3. We know that, the mass of a carbon atom is 12 u, mass of a hydrogen atom is 1 u and that of an oxygen atom is 16 u
4. So mass of a glucose molecule = (6 × 12 u) + (12 × 1 u) + (6 × 16 u) = 72 u + 12 u + 96 u = 180 u
5. Above we found that, to get NA glucose molecules, we must take 180 grams of glucose
• In (4) we have 180 u.
• In (5) we have 180 grams
• The numeric parts are the same
So we can write:
• Each molecule (it may be 'a molecule of an element' or 'a molecule of a compound') will have a unique value for the 'mass in grams'
• If we take that much mass of that element/compound, there will be NA number of molecules
• This unique mass is called 'Gram Molecular Mass' (GMM) of that molecule
Some examples:
• GMM of element oxygen is 16 grams
♦ In 16 grams of oxygen, there will be NA molecules of oxygen
• GMM of element helium is 4 grams
♦ In 4 grams of helium, there will be NA molecules of helium
• GMM of compound glucose is 180 grams
♦ In 180 grams of glucose, there will be NA molecules of glucose
Step 2: Write the numeric part
Step 3: Write 'grams' on the right side
This will give the GMM of that molecule
Solution:
Step 1: Total mass of one molecule:
Mass of one nitrogen atom = 14 u
Mass of one hydrogen atom = 1 u
So total mass = (1 × 14 u) + (3 × 1 u) = 14 u + 3 u = 17 u
Step 2: Write the numeric part: 17
Step 3: Write 'grams' on the right side. We get: 17 grams
So GMM of ammonia is 17 grams
1. Find the GMM of HCl
Solution:
Step 1: Total mass of one molecule:
Mass of one hydrogen atom = 1 u
Mass of one chlorine atom = 35.5 u
So total mass = (1 × 1 u) + (1 × 35.5 u) = 1 u + 35.5 u = 36.5 u
Step 2: Write the numeric part: 36.5
Step 3: Write 'grams' on the right side. We get: 36.5 grams
So GMM of ammonia is 36.5 grams
Solved example 10.1
How many water molecules are present in 90 grams of water?
Solution:
1. GMM of water (H2O) = (2 × 1) + (1 × 16) = 2 + 16 = 18 grams
2. No. of GMMs in 90 grams of water = 90⁄18 = 5
3. 1 GMM will contain NA molecules.
4. So no. of molecules in 5 GMMs = 5NA = 5 × 6.022×1023
No. of molecules × Mass (in grams) of one molecule = Given total Mass (in grams)
⇒ No. of molecules = Given total Mass (in grams)⁄Mass (in grams) of one molecule.
2. Given total mass = 90 grams
3. Mass (in u) of one molecule of water = (2 × 1) + (1 × 16) = 2 + 16 = 18 u
4. But 1 u = 1.6605×10-24 grams (Details here)
So 18 u = 18 × 1.6605×10-24 grams
5. Substituting this value in (1) we get:
■ We get the same result 5 × 6.022×1023 that we got earlier.
In these types of problems, it is worthy to always remember that, the reciprocal of 'u in grams' gives the Avogadro number NA. That is:

We can check it using a calculator
(i) 85 grams of ammonia (NH3)
(ii) 90 grams of glucose (C6H12O6)
(iii) 88 grams of carbon dioxide (CO2)
(iv) 50 grams of hydrogen (H2)
• Find the number of molecules in each sample. (Hint: GAMs are: H = 1g, C = 12 g, N = 14 g, O = 16 g)
Solution (i):
1. GMM of ammonia (NH3) = (1 × 14) + (1 × 3) = 14 + 3 = 17 grams
Note: For this step we require the atomic masses (in u) of nitrogen and hydrogen. But they are given to us in the form of GAM. We know that, GAM is numerically same as u.
2. No. of GMMs in 85 grams of water = 85⁄17 = 5
3. 1 GMM will contain NA molecules.
4. So no. of molecules in 5 GMMs = 5NA = 5 × 6.022×1023
No. of molecules × Mass (in grams) of one molecule = Given total Mass (in grams)
⇒ No. of molecules = Given total Mass (in grams)⁄Mass (in grams) of one molecule.
2. Given total mass = 85 grams
3. Mass (in u) of one molecule of water = (1 × 14) + (1 × 3) = 14 + 3 = 17 u
4. But 1 u = 1.6605×10-24 grams (Details here)
So 18 u = 18 × 1.6605×10-24 grams
5. Substituting this value in (1) we get:
■ We get the same result 5 × 6.022×1023 that we got earlier.
Solution (ii):
1. GMM of glucose (C6H12O6) = (6 × 12) + (1 × 12) + (6 × 16) = 72 + 12 + 96 = 180 grams
2. No. of GMMs in 90 grams of water = 90⁄180 = 0.5
3. 1 GMM will contain NA molecules.
4. So no. of molecules in 0.5 GMMs = 0.5NA = 0.5 × 6.022×1023
No. of molecules × Mass (in grams) of one molecule = Given total Mass (in grams)
⇒ No. of molecules = Given total Mass (in grams)⁄Mass (in grams) of one molecule.
2. Given total mass = 90 grams
3. Mass (in u) of one molecule of glucose = (6 × 12) + (1 × 12) + (6 × 16) = 72 + 12 + 96 = 180 u
4. But 1 u = 1.6605×10-24 grams (Details here)
So 180 u = 180 × 1.6605×10-24 grams
5. Substituting this value in (1) we get:
■ We get the same result 0.5 × 6.022×1023 that we got earlier.
Solution (iii):
1. GMM of carbon dioxide (CO2) = (1 × 12) + (16 × 2) = 12 + 32 = 44 grams
2. No. of GMMs in 88 grams of water = 88⁄44 = 2
3. 1 GMM will contain NA molecules.
4. So no. of molecules in 2 GMMs = 2NA = 2 × 6.022×1023
No. of molecules × Mass (in grams) of one molecule = Given total Mass (in grams)
⇒ No. of molecules = Given total Mass (in grams)⁄Mass (in grams) of one molecule.
2. Given total mass = 88 grams
3. Mass (in u) of one molecule of carbon dioxide = (1 × 12) + (16 × 2) = 12 + 32 = 44 u
4. But 1 u = 1.6605×10-24 grams (Details here)
So 44 u = 44 × 1.6605×10-24 grams
5. Substituting this value in (1) we get:
■ We get the same result 2 × 6.022×1023 that we got earlier.
Solution (iv):
1. GMM of hydrogen (H2) = (2 × 1) = 2 grams
2. No. of GMMs in 50 grams of water = 50⁄2 = 25
3. 1 GMM will contain NA molecules.
4. So no. of molecules in 25 GMMs = 25NA = 25 × 6.022×1023
No. of molecules × Mass (in grams) of one molecule = Given total Mass (in grams)
⇒ No. of molecules = Given total Mass (in grams)⁄Mass (in grams) of one molecule.
2. Given total mass = 50 grams
3. Mass (in u) of one molecule of glucose = (2 × 1) = 2 u
4. But 1 u = 1.6605×10-24 grams (Details here)
So 2 u = 2 × 1.6605×10-24 grams
5. Substituting this value in (1) we get:
■ We get the same result 2 × 6.022×1023 that we got earlier.
Some examples:
• GMM of element oxygen is 16 grams
♦ In 16 grams of oxygen, there will be NA molecules of oxygen
• GMM of element helium is 4 grams
♦ In 4 grams of helium, there will be NA molecules of helium
• GMM of compound glucose is 180 grams
♦ In 180 grams of glucose, there will be NA molecules of glucose
The GMM of any molecule can be written using the following steps
Step 1: Calculate the total mass of one molecule. It's unit will be in 'u'Step 2: Write the numeric part
Step 3: Write 'grams' on the right side
This will give the GMM of that molecule
Some examples:
1. Find the GMM of ammonia (NH3)Solution:
Step 1: Total mass of one molecule:
Mass of one nitrogen atom = 14 u
Mass of one hydrogen atom = 1 u
So total mass = (1 × 14 u) + (3 × 1 u) = 14 u + 3 u = 17 u
Step 2: Write the numeric part: 17
Step 3: Write 'grams' on the right side. We get: 17 grams
So GMM of ammonia is 17 grams
1. Find the GMM of HCl
Solution:
Step 1: Total mass of one molecule:
Mass of one hydrogen atom = 1 u
Mass of one chlorine atom = 35.5 u
So total mass = (1 × 1 u) + (1 × 35.5 u) = 1 u + 35.5 u = 36.5 u
Step 2: Write the numeric part: 36.5
Step 3: Write 'grams' on the right side. We get: 36.5 grams
So GMM of ammonia is 36.5 grams
Once we understand the basics, there will not be any need to write the steps. Just write the total mass of one molecule, and write 'grams' instead of 'u'
We will now see some solved examplesSolved example 10.1
How many water molecules are present in 90 grams of water?
Solution:
1. GMM of water (H2O) = (2 × 1) + (1 × 16) = 2 + 16 = 18 grams
2. No. of GMMs in 90 grams of water = 90⁄18 = 5
3. 1 GMM will contain NA molecules.
4. So no. of molecules in 5 GMMs = 5NA = 5 × 6.022×1023
Let us do the above problem by another method, which uses some very basic information:
1. We have:No. of molecules × Mass (in grams) of one molecule = Given total Mass (in grams)
⇒ No. of molecules = Given total Mass (in grams)⁄Mass (in grams) of one molecule.
2. Given total mass = 90 grams
3. Mass (in u) of one molecule of water = (2 × 1) + (1 × 16) = 2 + 16 = 18 u
4. But 1 u = 1.6605×10-24 grams (Details here)
So 18 u = 18 × 1.6605×10-24 grams
5. Substituting this value in (1) we get:
In these types of problems, it is worthy to always remember that, the reciprocal of 'u in grams' gives the Avogadro number NA. That is:

We can check it using a calculator
Solved example 10.2
Samples of some compounds are given:(i) 85 grams of ammonia (NH3)
(ii) 90 grams of glucose (C6H12O6)
(iii) 88 grams of carbon dioxide (CO2)
(iv) 50 grams of hydrogen (H2)
• Find the number of molecules in each sample. (Hint: GAMs are: H = 1g, C = 12 g, N = 14 g, O = 16 g)
Solution (i):
1. GMM of ammonia (NH3) = (1 × 14) + (1 × 3) = 14 + 3 = 17 grams
Note: For this step we require the atomic masses (in u) of nitrogen and hydrogen. But they are given to us in the form of GAM. We know that, GAM is numerically same as u.
2. No. of GMMs in 85 grams of water = 85⁄17 = 5
3. 1 GMM will contain NA molecules.
4. So no. of molecules in 5 GMMs = 5NA = 5 × 6.022×1023
Let us do the problem by another method, which uses some very basic information:
1. We have:No. of molecules × Mass (in grams) of one molecule = Given total Mass (in grams)
⇒ No. of molecules = Given total Mass (in grams)⁄Mass (in grams) of one molecule.
2. Given total mass = 85 grams
3. Mass (in u) of one molecule of water = (1 × 14) + (1 × 3) = 14 + 3 = 17 u
4. But 1 u = 1.6605×10-24 grams (Details here)
So 18 u = 18 × 1.6605×10-24 grams
5. Substituting this value in (1) we get:
■ We get the same result 5 × 6.022×1023 that we got earlier.
Solution (ii):
1. GMM of glucose (C6H12O6) = (6 × 12) + (1 × 12) + (6 × 16) = 72 + 12 + 96 = 180 grams
2. No. of GMMs in 90 grams of water = 90⁄180 = 0.5
3. 1 GMM will contain NA molecules.
4. So no. of molecules in 0.5 GMMs = 0.5NA = 0.5 × 6.022×1023
Let us do the above problem by the other method:
1. We have:No. of molecules × Mass (in grams) of one molecule = Given total Mass (in grams)
⇒ No. of molecules = Given total Mass (in grams)⁄Mass (in grams) of one molecule.
2. Given total mass = 90 grams
3. Mass (in u) of one molecule of glucose = (6 × 12) + (1 × 12) + (6 × 16) = 72 + 12 + 96 = 180 u
4. But 1 u = 1.6605×10-24 grams (Details here)
So 180 u = 180 × 1.6605×10-24 grams
5. Substituting this value in (1) we get:
■ We get the same result 0.5 × 6.022×1023 that we got earlier.
Solution (iii):
1. GMM of carbon dioxide (CO2) = (1 × 12) + (16 × 2) = 12 + 32 = 44 grams
2. No. of GMMs in 88 grams of water = 88⁄44 = 2
3. 1 GMM will contain NA molecules.
4. So no. of molecules in 2 GMMs = 2NA = 2 × 6.022×1023
Let us do the above problem by the other method:
1. We have:No. of molecules × Mass (in grams) of one molecule = Given total Mass (in grams)
⇒ No. of molecules = Given total Mass (in grams)⁄Mass (in grams) of one molecule.
2. Given total mass = 88 grams
3. Mass (in u) of one molecule of carbon dioxide = (1 × 12) + (16 × 2) = 12 + 32 = 44 u
4. But 1 u = 1.6605×10-24 grams (Details here)
So 44 u = 44 × 1.6605×10-24 grams
5. Substituting this value in (1) we get:
■ We get the same result 2 × 6.022×1023 that we got earlier.
Solution (iv):
1. GMM of hydrogen (H2) = (2 × 1) = 2 grams
2. No. of GMMs in 50 grams of water = 50⁄2 = 25
3. 1 GMM will contain NA molecules.
4. So no. of molecules in 25 GMMs = 25NA = 25 × 6.022×1023
Let us do the above problem by the other method:
1. We have:No. of molecules × Mass (in grams) of one molecule = Given total Mass (in grams)
⇒ No. of molecules = Given total Mass (in grams)⁄Mass (in grams) of one molecule.
2. Given total mass = 50 grams
3. Mass (in u) of one molecule of glucose = (2 × 1) = 2 u
4. But 1 u = 1.6605×10-24 grams (Details here)
So 2 u = 2 × 1.6605×10-24 grams
5. Substituting this value in (1) we get:
■ We get the same result 2 × 6.022×1023 that we got earlier.
Based on the above discussion, we can now take up 'mole'. We will see it in the next section.




