In the previous section, we saw reason for the displacement of silver from a solution of silver nitrate. In this section, we will see how metals can be used to make electricity.
1. Take two beakers. Add 100 mL of 1 M zinc sulphate solution into one beaker.
2. Add 100 mL of copper sulphate solution into the other. See fig.12.5 below:
[We have seen the method to prepare 1 M solution of any given salt. See details here]
3. Immerse a zinc rod in the zinc sulphate solution.
4. Immerse a copper rod in the copper sulphate solution.
5. Connect the negative terminal of a voltmeter to the zinc rod. Connect the positive terminal to the copper rod.
6. Connect the solutions in the two beakers by a salt bridge. A long strip of filter paper soaked in KCl solution can be used instead of the salt bridge.
7. Now observe the change in the voltmeter reading.
From the voltmeter, it is clear that electricity is produced in the experiment. Let us see the reason:
8. In the previous experiments we have seen that zinc is more reactive than copper.
So Zn in the zinc rod, loses two electrons and become. Zn2+. The equation can be written as:
Zn0 (s) ⟶ Zn+2 (aq) + 2e-1
• This is a oxidation reaction. Because the oxidation number of Zn increases from zero to +2.
• In other words:
Losing electrons is oxidation. So an oxidation reaction is taking place here.
■ The electrode at which oxidation takes place is called the anode.
9. The newly formed Zn+2 ions gets detached from the zinc rod and goes into the solution. But the released electrons stick to the Zn rod
• Note that many Zn+2 ions are already present in the zinc sulphate solution because, the aqueous zinc sulphate solution exists as a mixture of Zn+2 ions and (SO4)-2 ions.
10. Because of the released electrons, the Zn rod becomes negatively charged. These free electrons reach the copper rod through the external circuit
11. These electrons which reach the copper rod flows through the copper rod and reaches the copper sulphate solution.
• In the copper solution, Cu+2 ions are present. These ions receive the electrons and become Cu atoms. The equation can be written as:
Cu+2 (aq) + 2e-1 ⟶ Cu0 (s)
• This is a reduction reaction. Because the oxidation number of Cu+2 decreases from +2 to zero.
• In other words:
Gaining electrons is reduction. So a reduction reaction is taking place here.
■ The electrode at which reduction takes place is called the cathode.
12. So both oxidation and reduction takes place in this reaction. Thus it is a redox reaction.
• The transfer of electrons produced by the redox reaction causes the flow of electric current.
• If a flow of electric current is obtained from a device, we can call it a cell.
• The cell which converts chemical energy to electrical energy through redox reaction is called Galvanic cell or Voltaic cell.
• They are named after the scientists Luigi Galvani and Alessandro Volta who achieved early developments in such cells.
• Out of the two metals zinc and copper, zinc is more ready to donate electrons.
• So oxidation takes place at the zinc rod.
• We have seen that, the electrode at which oxidation takes place is called the anode.
So we can say this:
■ In the galvanic cell, the more reactive metal will become the anode
The opposite can also be written:
■ In a galvanic cell, the less reactive metal will become the cathode
Fact 2:
• We have seen that the free electrons are first formed when the Zn atoms become Zn+2 ions.
• This happens at the anode.
■ Thus in a galvanic cell, the electrons flow is from the anode to cathode
The above facts are shown in the fig.12.6 below:
• Metals like potassium, sodium, zinc, copper etc., also undergo corrosion.
♦ Atoms of the anode loses electrons and become ions.
• If in the place of copper, we use a metal which is more reactive than zinc, then that metal will become anode.
♦ Zinc will become the cathode and will be saved.
• So we must make the metal to be protected, the cathode
• This is the basis of cathodic protection.
■ Cathodic protection is a technique used to control the corrosion of a metal surface by making it the cathode of an electrochemical cell. In the simplest form, the metal to be protected is connected to a more easily corroded 'sacrificial metal' to act as the anode
• We will see more technical details of this method in higher classes
Now we will discuss about the salt bridge
• Consider the cathode compartment in the above galvanic cell.
♦ We have a copper rod in a copper sulphate solution.
• The copper sulphate solution always exists as a mixture of Cu+2 and (SO4)-2 ions.
• We have seen that, the electrons reaching the copper rod will be received by the Cu+2 ions, and they will become Cu atoms.
• So the positive charges in the solution will continuously decrease. In other words, there will be an excess negative charge (due to the (SO4)-2 ions) in the solution.
• So these ions flow from the copper compartment to the zinc compartment through the salt bridge.
• This will help to maintain the electrical neutrality of the solution
So we have completed this discussion on Galvanic cell using Zinc and copper. In the next section, we will see another Galvanic cell.
1. Take two beakers. Add 100 mL of 1 M zinc sulphate solution into one beaker.
2. Add 100 mL of copper sulphate solution into the other. See fig.12.5 below:
[We have seen the method to prepare 1 M solution of any given salt. See details here]
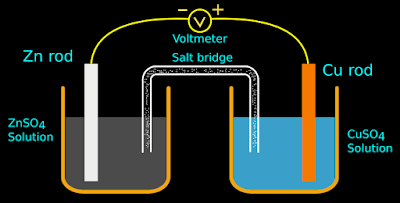 |
| Fig.12.5 |
4. Immerse a copper rod in the copper sulphate solution.
5. Connect the negative terminal of a voltmeter to the zinc rod. Connect the positive terminal to the copper rod.
6. Connect the solutions in the two beakers by a salt bridge. A long strip of filter paper soaked in KCl solution can be used instead of the salt bridge.
■ Method of making a salt bridge:
• Make a paste by mixing two items: (i) gelatin (ii) Potassium chloride (KCl)
♦ agar agar gel can be used instead of gelatin
♦ Potassium nitrate (KNO3) or Ammonium chloride (NH4Cl) can be used instead of KCl
• Fill this paste into an U-tube
• Close the ends of the tube with cotton balls
• The salt bridge is ready to use. We will see it's function later in this section
|
|---|
From the voltmeter, it is clear that electricity is produced in the experiment. Let us see the reason:
8. In the previous experiments we have seen that zinc is more reactive than copper.
So Zn in the zinc rod, loses two electrons and become. Zn2+. The equation can be written as:
Zn0 (s) ⟶ Zn+2 (aq) + 2e-1
• This is a oxidation reaction. Because the oxidation number of Zn increases from zero to +2.
• In other words:
Losing electrons is oxidation. So an oxidation reaction is taking place here.
■ The electrode at which oxidation takes place is called the anode.
9. The newly formed Zn+2 ions gets detached from the zinc rod and goes into the solution. But the released electrons stick to the Zn rod
• Note that many Zn+2 ions are already present in the zinc sulphate solution because, the aqueous zinc sulphate solution exists as a mixture of Zn+2 ions and (SO4)-2 ions.
10. Because of the released electrons, the Zn rod becomes negatively charged. These free electrons reach the copper rod through the external circuit
11. These electrons which reach the copper rod flows through the copper rod and reaches the copper sulphate solution.
• In the copper solution, Cu+2 ions are present. These ions receive the electrons and become Cu atoms. The equation can be written as:
Cu+2 (aq) + 2e-1 ⟶ Cu0 (s)
• This is a reduction reaction. Because the oxidation number of Cu+2 decreases from +2 to zero.
• In other words:
Gaining electrons is reduction. So a reduction reaction is taking place here.
■ The electrode at which reduction takes place is called the cathode.
12. So both oxidation and reduction takes place in this reaction. Thus it is a redox reaction.
• The transfer of electrons produced by the redox reaction causes the flow of electric current.
• If a flow of electric current is obtained from a device, we can call it a cell.
• The cell which converts chemical energy to electrical energy through redox reaction is called Galvanic cell or Voltaic cell.
• They are named after the scientists Luigi Galvani and Alessandro Volta who achieved early developments in such cells.
In such cells, we can see two interesting facts:
Fact 1:• Out of the two metals zinc and copper, zinc is more ready to donate electrons.
• So oxidation takes place at the zinc rod.
• We have seen that, the electrode at which oxidation takes place is called the anode.
So we can say this:
■ In the galvanic cell, the more reactive metal will become the anode
The opposite can also be written:
■ In a galvanic cell, the less reactive metal will become the cathode
Fact 2:
• We have seen that the free electrons are first formed when the Zn atoms become Zn+2 ions.
• This happens at the anode.
■ Thus in a galvanic cell, the electrons flow is from the anode to cathode
The above facts are shown in the fig.12.6 below:
Corrosion of metals
At this stage, we can have a short discussion about the basics of 'corrosion of metals'.
• We have seen that the zinc atoms become Zn+2 ions and leave the zinc rod. If the process continues, the zinc rod will soon become useless.
• The zinc atoms left the rod because favorable conditions were available on the surface of the zinc rod.
♦ We provided those favorable conditions by connecting it with a copper rod, providing zinc sulphate and copper sulphate solutions etc.,
• If such favorable conditions occur naturally around the surface of any metal, it's atoms would surely leave.
• For example, the oxygen and water vapour present in the atmospheric air can provide favorable conditions for certain metals.
• The atoms of those metals will then become ions and will leave the original metal surface.
• The released electrons will be received by oxygen. The oxygen become negative ions.
• The 'positive metal ions' and 'negative oxygen ions' together will form a new compound. The rust which we see on the surface of iron is such a compound.
■ The process of conversion of a metal into it's compounds by continuous interaction with atmospheric air and water vapour is termed corrosion of metals. This is an electrochemical reaction. • The released electrons will be received by oxygen. The oxygen become negative ions.
• The 'positive metal ions' and 'negative oxygen ions' together will form a new compound. The rust which we see on the surface of iron is such a compound.
• Metals like potassium, sodium, zinc, copper etc., also undergo corrosion.
Cathodic protection
• In the above discussion, we have seen that the zinc which is more reactive than copper has become the anode.♦ Atoms of the anode loses electrons and become ions.
• If in the place of copper, we use a metal which is more reactive than zinc, then that metal will become anode.
♦ Zinc will become the cathode and will be saved.
• So we must make the metal to be protected, the cathode
• This is the basis of cathodic protection.
■ Cathodic protection is a technique used to control the corrosion of a metal surface by making it the cathode of an electrochemical cell. In the simplest form, the metal to be protected is connected to a more easily corroded 'sacrificial metal' to act as the anode
• We will see more technical details of this method in higher classes
• Consider the cathode compartment in the above galvanic cell.
♦ We have a copper rod in a copper sulphate solution.
• The copper sulphate solution always exists as a mixture of Cu+2 and (SO4)-2 ions.
• We have seen that, the electrons reaching the copper rod will be received by the Cu+2 ions, and they will become Cu atoms.
• So the positive charges in the solution will continuously decrease. In other words, there will be an excess negative charge (due to the (SO4)-2 ions) in the solution.
• So these ions flow from the copper compartment to the zinc compartment through the salt bridge.
• This will help to maintain the electrical neutrality of the solution
So we have completed this discussion on Galvanic cell using Zinc and copper. In the next section, we will see another Galvanic cell.

No comments:
Post a Comment