In the previous section, we completed a discussion on nomenclature of hydrocarbons. In this section we will discuss some chemical reactions involving hydrocarbons.
• In organic chemistry, we deal with the following three topics:
♦ Study of different hydrocarbons
♦ Study of different compounds obtained from hydrocarbons
♦ Study of the different chemical reactions in which hydrocarbons are reactants or products
• Many substances that we use in our day to day life, and also many substances which are essential in scientific and engineering fields, are contributions of organic chemistry.
• Medicines, polymers, fuels etc., are examples
• Such substances are obtained through different chemical reactions.
• In this chapter we will discuss some of those basic chemical reactions.
• Note that, the reaction will take place in the presence of sunlight because, sunlight is required to break the bonds between atoms
Stage 1:
• We know that, in methane, there are 4 hydrogen atoms around a carbon atom.
• In stage 1, one of those hydrogen atoms will be removed and a chlorine atom will take it's place.
• So the original methane will become chloromethane.
• The removed hydrogen atom will combine with a chlorine atom to become hydrogen chloride (HCl).
• This is shown in fig.15.1 below:
Stage 2:
• In stage 2, one hydrogen atom from the newly formed chloromethane will be removed and a chlorine atom will take it's place.
• So the original chloromethane will become dichloromethane. The prefix 'di' is used to indicate two chlorine atoms in the molecule.
• So in dichloromethane, there are 2 hydrogen atoms and 2 chlorine atoms around a carbon atom.
• The removed hydrogen atom will combine with a chlorine atom to become hydrogen chloride (HCl).
• This is shown in fig.15.2 below:
Stage 3:
• In stage 3, one hydrogen atom from the newly formed dichloromethane will be removed and a chlorine atom will take it's place.
• So the original dichloromethane will become trichloromethane. The prefix 'tri' is used to indicate three chlorine atoms in the molecule. Trichloromethane is also called chloroform
• So in trichloromethane, there are 1 hydrogen atom and 3 chlorine atoms around a carbon atom.
• The removed hydrogen atom will combine with a chlorine atom to become hydrogen chloride (HCl).
• This is shown in fig.15.3 below:
Stage 4:
• In stage 4, one remaining hydrogen atom from the newly formed trichloromethane will be removed and a chlorine atom will take it's place.
• So the original trichloromethane will become tetrachloromethane. The prefix 'tetra' is used to indicate four chlorine atoms in the molecule. Tetrachloromethane is also called carbon tetrachloride
• So in tetrachloromethane, there 4 chlorine atoms around a carbon atom. There are no hydrogen atoms.
• The removed hydrogen atom will combine with a chlorine atom to become hydrogen chloride (HCl).
• This is shown in fig.15.4 below:
• The above figs., shows the structural formulae. The same reactions can be written using molecular formulae as follows:
Stage 1: CH4 + Cl2 → CH3Cl + HCl
Stage 2: CH3Cl + Cl2 → CH2Cl2 + HCl
Stage 3: CH2Cl2 + Cl2 → CHCl3 + HCl
Stage 4: CHCl3 + Cl2 → CCl4 + HCl
• In the above reaction, in each stage, one hydrogen atom is replaced by a chlorine atom.
Reactions in which an atom or a group in a compound is replaced by another atom or a group are called substitution reactions.
• Substitution reactions between ethane and chlorine can be written as follows:
Stage 1: C2H6 + Cl2 → C2H5Cl + HCl
Stage 2: C2H5Cl + Cl2 → C2H4Cl2 + HCl
Stage 3: C2H4Cl2 + Cl2 → C2H3Cl3 + HCl
Stage 4: C2H3Cl3 + Cl2 → C2H2Cl4 + HCl
Stage 5: C2H2Cl4 + Cl2 → C2HCl5 + HCl
Stage 6: C2HCl5 + Cl2 → C2Cl6 + HCl
• We also know that in ethene, one double bond is present. It is an unsaturated compound.
• They are shown in fig.15.5 below:
• Fig.15.5(a) is an ethane molecule. Fig.(b) is an ethene molecule
• When unsaturated compounds take part in chemical reactions, they tend to form saturated compounds. Let us check if this is true for ethene:
• Ethene reacts with hydrogen at high temperature in the presence of nickel catalyst.
• The product formed is ethane, which is a saturated compound. The reaction is shown below:
• Note that, the two carbon atoms no longer need to maintain a double bond between them, as the two new hydrogen atoms will supply the required electrons
• Using molecular formulae, it can be written as:
C2H4 + H2 → C2H6
Another example:
• In the above reaction, we have propene and chlorine on the left side.
• Propene is an unsaturated compound. It reacts with chlorine to form 1,2 - Dichloropropane, which is a saturated compound
• This can be written in another form also:
CH3ㅡCH=CH2 + Cl2 → C3H6Cl2
• Note that, the two carbon atoms no longer need to maintain a double bond between them, as the two new chlorine atoms will supply the required electrons
• We have already seen in the previous chapter, how a chloro group gets itself attached to a carbon chain (Details here)
Some more examples:
(i) CH2=CH2 + Cl2 → C2H4Cl2
The structural formulae are shown below:
The product is Dichloroethane. Note that, it has all single bonds
(ii) CH2=CH2 + HCl → C2H5Cl
The structural formulae are shown below:
The product is Chloroethane. Note that, it has all single bonds
(iii) CH3ㅡCH=CH2 + H2 → C3H8
The structural formulae are shown below:
The product is propane. Note that, it has all single bonds
(iv) CH3ㅡCH=CHㅡCH3 + HBr → C4H9Br
The structural formulae are shown below:
The product is 2-bromobutane.
• Note that, the new hydrogen and bromine atoms cannot take up 'any position they like' in the But-2-ene.
♦ The new hydrogen atom has to be attached to one of the two carbon atoms in the double bond in But-2-ene
♦ The new bromine atom has to be attached to the other carbon atom in the double bond in But-2-ene
■ So we find that, compounds with double bond changes to compounds with all single bonds.
• In a similar way, compounds with triple bonds can also change to compounds with all single bonds.
• This will take place in stages.
♦ In the first stage, the triple bond in an alkyne changes to a double bond.
♦ In the second stage, the double bond changes to a single bond.
• The example of ethyne is given below:
Stage 1: Ethyne changes to ehene:
CH ☰ CH + H2 → C2H2
Stage 2: Ethene changes to ethane:
CH2=CH2 + H2 → C2H6
Reactions in which unsaturated organic compounds with double bond or triple bond react with other molecules to form saturated compounds are called addition reactions.
• In addition reactions, the original unsaturated molecule combine with a 'molecule of another compound' to become saturated.
♦ This 'another compound' can be hydrogen, chlorine, hydrogen chloride, hydrogen bromide etc.,
• Now we will see the change from unsaturated to saturated, with out any help from 'another compound'.
♦ That is., the molecules of some unsaturated compound will combine among themselves to form saturated compounds.
■ Let us see an example.
• Consider an ethene molecule. It wants to become a saturated molecule by combining with another ethene molecule. Is it possible? Let us see:
We will write the steps:
1. In fig.15.12(a) below, two individual ethene molecules are shown.
• All the valency requirements are satisfied and each of them are stable individually.
2. Now, each of them individually decides to discard the double bond and 'maintain a single bond only' between their two carbon atoms. The result is shown in fig.15.12(b)
3. But as we can see, there are unsatisfied bonds. They are shown inside small red circles.
• There are a total of four unsatisfied bonds. They are called 'unsatisfied' because, such bonds have only one electron.
• This is shown more clearly in the electron dot diagram in fig.15.13(a) below:
• Consider any one carbon atom in fig.15.7(a). There are only 7 electrons around it:
♦ 5 green and two red.
• So one more electron is required.
4. Consider fig.15.13(b)
• The two molecules have decided to combine together.
• So the two carbon atoms in the middle will bond together. Thus a larger molecule is formed.
• The result is shown in fig.15.14(a) below:
5. In fig.14.14(a), the larger molecule consists of two simpler molecules of ethene.
• Even when this larger molecule is formed from two smaller molecules, the outer two carbon atoms still need one electron each. We can represent it as: ㅡ[CH2ㅡCH2]2ㅡ
• 'CH2ㅡCH2' is one ethene molecule. Putting it inside square brackets and giving a subscript '2' indicates that two ethene molecules are combined together inside the square brackets.
• The 'ㅡ' symbol at both ends indicate that, even after the combination, unsatisfied bond exists at the ends
6. Obviously, another two such simple molecules of ethene can be attached at the ends. This is shown by the ellipses in fig.15.8(b).
• The resulting larger molecule can be represented as: ㅡ[CH2ㅡCH2]4ㅡ .
7. In this way a long chain can be formed with a large number of ethene molecules.
• The structure of the chain is shown in fig.15.15 below:
• This structure can be represented as: ㅡ[CH2ㅡCH2]nㅡ
♦ Where 'n' is a very large number
Polymerisation is the process in which a large number of simple molecules combine under suitable conditions to form complex molecules.
• The complex molecules thus formed are called polymers.
• The simple molecules which combine in this manner are called monomers.
• We use a number of polymers in our daily life.
♦ Some of them are natural polymers
♦ Others are man-made polymers
■ In the example that we saw above, the monomer is ethene
■ The IUPAC name of the polymer is polyethene
• It is commonly known as polythene.
• It is also known as polyethylene. Since ethylene was the common name for ethene before IUPAC names were implemented.
• It is the most common type of plastic, and is used for making plastic bags, bottles etc.,
■ The IUPAC name of another important polymer is: Poly(1-chloroethene)
• From this IUPAC name, we get the following information:
(i) The monomer is written inside the brackets. It is: 1-chloroethene
(ii) '1-chloroethene' indicates that it is an alkene. In this case, the alkene is obviously ethene.
(iii) One hydrogen atom in the ethene is replaced by a chlorine atom. Hence the name: 1-chloroethene
(iv) So the monomer is: CH2=CHCl. It's structure is shown in fig.15.16(a) below.
• But the double bond between the two carbon atoms breaks and become a single bond.
♦ So it can be represented as: ㅡ[CH2ㅡCHCl]ㅡ
♦ It's structure is shown in fig.15.16(b) below.
• Now it is ready to form the chain. The chain can be represented as: ㅡ[CH2ㅡCHCl]nㅡ
♦ It's structure can be represented as shown in fig.15.16(c) below:
[We have already seen in the previous chapter, how a chloro group gets itself attached to a carbon chain (Details here). We saw it again above in this section in addition reaction. So the electron dot diagram of the chain is not drawn here. However, the reader may draw it in his/her notebooks]
• The monomer '1-chloroethene', or simply 'chloroethene', is commonly known as vinylchloride
• So when the monomer vinylchloride becomes a polymer, it's common name will be polyvinylchloride.
• It is popularly known as PVC, and is used for making pipes, electric cable insulation etc.,
■ The IUPAC name of another important polymer is: Poly(1,1,2,2-tetrafluoroethene)
• From this IUPAC name, we get the following information:
(i) The monomer is written inside the brackets. It is: 1,1,2,2-tetrafluoroethene
(ii) '1,1,2,2-tetrafluoroethene' indicates that it is an alkene. In this case, the alkene is obviously ethene.
(iii) One ethene molecule has four hydrogen atoms. All those hydrogen atoms are replaced by fluorine atoms. Hence the name: 1,1,2,2-tetrafluoroethene
(iv) So the monomer is: CF2=CF2. It's structure is shown in fig.15.17(a) below.
• But the double bond between the two carbon atoms breaks and become a single bond.
♦ So it can be represented as: ㅡ[CF2ㅡCF2]ㅡ
♦ It's structure is shown in fig.15.17(b) below.
• Now it is ready to form the chain. The chain can be represented as: ㅡ[CF2ㅡCF2]nㅡ
♦ It's structure can be represented as shown in fig.15.17(c) below:
[We have already seen in the previous chapter, how a chloro group gets itself attached to a carbon chain (Details here). We saw it again above in this section in addition reaction. Fluorine and chlorine belongs to the same halogen family. Their valencies are the same. So the electron dot diagram of the chain is not drawn here. However, the reader may draw it in his/her notebooks]
• It is used for coating the inner surface of non-stick cookware.
■ The IUPAC name of yet another important polymer is: Poly(propene)
• From this IUPAC name, we get the following information:
(i) The monomer is written inside the brackets. It is: propene
(ii) We know that 'propene' is an alkene. It's formula is: CH3ㅡCH=CH2
(iii) It's structure is shown in fig.15.18(a) below.
• But the double bond between the two carbon atoms breaks and become a single bond.
But it cannot be represented as: ㅡ[CH3ㅡCHㅡCH2]ㅡ
Let us analyse the reason:
(i) Till now we have seen monomers with upto two carbon atoms only.
(ii) But our present monomer propene has 3 carbon atoms.
iii) So while writing the structure, the carbon atoms on either sides of the double bond should come in a straight line.
(iv) This is because, the 'unsatisfied bonds' will be starting from the carbon atoms on either sides of the double bond
(v) The third carbon atom should be written above or below the line. This is shown in fig.15.18(b) below:
[As in the previous two cases, the reader may draw the electron dot diagram in his/her own notebooks]
• The propene is commonly known as propylene
• So when the monomer propylene becomes a polymer, it's common name will be polypropylene
• It is used for making table tops and other utensils which require thermal resistance.
■ It is interesting to note that rubber (natural and man-made) is a polymer. The monomer in this case is isoprene
• Isoprene is an alkene having two double bonds. We will see it's polymer structure in higher classes
In the next section, we will see some more reactions.
• In organic chemistry, we deal with the following three topics:
♦ Study of different hydrocarbons
♦ Study of different compounds obtained from hydrocarbons
♦ Study of the different chemical reactions in which hydrocarbons are reactants or products
• Many substances that we use in our day to day life, and also many substances which are essential in scientific and engineering fields, are contributions of organic chemistry.
• Medicines, polymers, fuels etc., are examples
• Such substances are obtained through different chemical reactions.
• In this chapter we will discuss some of those basic chemical reactions.
Substitution reaction
First let us see the reaction between methane (CH4) and chlorine in the presence of sunlight. This reaction can be analyzed in stages:• Note that, the reaction will take place in the presence of sunlight because, sunlight is required to break the bonds between atoms
Stage 1:
• We know that, in methane, there are 4 hydrogen atoms around a carbon atom.
• In stage 1, one of those hydrogen atoms will be removed and a chlorine atom will take it's place.
• So the original methane will become chloromethane.
• The removed hydrogen atom will combine with a chlorine atom to become hydrogen chloride (HCl).
• This is shown in fig.15.1 below:
 |
| Fig.15.1 |
• In stage 2, one hydrogen atom from the newly formed chloromethane will be removed and a chlorine atom will take it's place.
• So the original chloromethane will become dichloromethane. The prefix 'di' is used to indicate two chlorine atoms in the molecule.
• So in dichloromethane, there are 2 hydrogen atoms and 2 chlorine atoms around a carbon atom.
• The removed hydrogen atom will combine with a chlorine atom to become hydrogen chloride (HCl).
• This is shown in fig.15.2 below:
 |
| Fig.15.2 |
• In stage 3, one hydrogen atom from the newly formed dichloromethane will be removed and a chlorine atom will take it's place.
• So the original dichloromethane will become trichloromethane. The prefix 'tri' is used to indicate three chlorine atoms in the molecule. Trichloromethane is also called chloroform
• So in trichloromethane, there are 1 hydrogen atom and 3 chlorine atoms around a carbon atom.
• The removed hydrogen atom will combine with a chlorine atom to become hydrogen chloride (HCl).
• This is shown in fig.15.3 below:
 |
| Fig.15.3 |
• In stage 4, one remaining hydrogen atom from the newly formed trichloromethane will be removed and a chlorine atom will take it's place.
• So the original trichloromethane will become tetrachloromethane. The prefix 'tetra' is used to indicate four chlorine atoms in the molecule. Tetrachloromethane is also called carbon tetrachloride
• So in tetrachloromethane, there 4 chlorine atoms around a carbon atom. There are no hydrogen atoms.
• The removed hydrogen atom will combine with a chlorine atom to become hydrogen chloride (HCl).
• This is shown in fig.15.4 below:
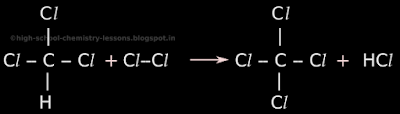 |
| Fig.15.4 |
Stage 1: CH4 + Cl2 → CH3Cl + HCl
Stage 2: CH3Cl + Cl2 → CH2Cl2 + HCl
Stage 3: CH2Cl2 + Cl2 → CHCl3 + HCl
Stage 4: CHCl3 + Cl2 → CCl4 + HCl
• In the above reaction, in each stage, one hydrogen atom is replaced by a chlorine atom.
Reactions in which an atom or a group in a compound is replaced by another atom or a group are called substitution reactions.
• Substitution reactions between ethane and chlorine can be written as follows:
Stage 1: C2H6 + Cl2 → C2H5Cl + HCl
Stage 2: C2H5Cl + Cl2 → C2H4Cl2 + HCl
Stage 3: C2H4Cl2 + Cl2 → C2H3Cl3 + HCl
Stage 4: C2H3Cl3 + Cl2 → C2H2Cl4 + HCl
Stage 5: C2H2Cl4 + Cl2 → C2HCl5 + HCl
Stage 6: C2HCl5 + Cl2 → C2Cl6 + HCl
Addition reaction
• We know that ethane has all single bonds. It is a saturated compound• We also know that in ethene, one double bond is present. It is an unsaturated compound.
• They are shown in fig.15.5 below:
 |
| Fig.15.5 |
• When unsaturated compounds take part in chemical reactions, they tend to form saturated compounds. Let us check if this is true for ethene:
• Ethene reacts with hydrogen at high temperature in the presence of nickel catalyst.
• The product formed is ethane, which is a saturated compound. The reaction is shown below:
 |
| Fig.15.6 |
• Using molecular formulae, it can be written as:
C2H4 + H2 → C2H6
Another example:
 |
| Fig.15.7 |
• Propene is an unsaturated compound. It reacts with chlorine to form 1,2 - Dichloropropane, which is a saturated compound
• This can be written in another form also:
CH3ㅡCH=CH2 + Cl2 → C3H6Cl2
• Note that, the two carbon atoms no longer need to maintain a double bond between them, as the two new chlorine atoms will supply the required electrons
• We have already seen in the previous chapter, how a chloro group gets itself attached to a carbon chain (Details here)
(i) CH2=CH2 + Cl2 → C2H4Cl2
The structural formulae are shown below:
 |
| Fig.15.8 |
(ii) CH2=CH2 + HCl → C2H5Cl
The structural formulae are shown below:
 |
| Fig.15.9 |
(iii) CH3ㅡCH=CH2 + H2 → C3H8
The structural formulae are shown below:
 |
| Fig.15.10 |
(iv) CH3ㅡCH=CHㅡCH3 + HBr → C4H9Br
The structural formulae are shown below:
 |
| Fig.15.11 |
• Note that, the new hydrogen and bromine atoms cannot take up 'any position they like' in the But-2-ene.
♦ The new hydrogen atom has to be attached to one of the two carbon atoms in the double bond in But-2-ene
♦ The new bromine atom has to be attached to the other carbon atom in the double bond in But-2-ene
■ So we find that, compounds with double bond changes to compounds with all single bonds.
• In a similar way, compounds with triple bonds can also change to compounds with all single bonds.
• This will take place in stages.
♦ In the first stage, the triple bond in an alkyne changes to a double bond.
♦ In the second stage, the double bond changes to a single bond.
• The example of ethyne is given below:
Stage 1: Ethyne changes to ehene:
CH ☰ CH + H2 → C2H2
Stage 2: Ethene changes to ethane:
CH2=CH2 + H2 → C2H6
Reactions in which unsaturated organic compounds with double bond or triple bond react with other molecules to form saturated compounds are called addition reactions.
Polymerisation
• From the discussion on addition reaction above, we find that the unsaturated compounds have a tendency to become saturated compounds.• In addition reactions, the original unsaturated molecule combine with a 'molecule of another compound' to become saturated.
♦ This 'another compound' can be hydrogen, chlorine, hydrogen chloride, hydrogen bromide etc.,
• Now we will see the change from unsaturated to saturated, with out any help from 'another compound'.
♦ That is., the molecules of some unsaturated compound will combine among themselves to form saturated compounds.
■ Let us see an example.
• Consider an ethene molecule. It wants to become a saturated molecule by combining with another ethene molecule. Is it possible? Let us see:
We will write the steps:
1. In fig.15.12(a) below, two individual ethene molecules are shown.
• All the valency requirements are satisfied and each of them are stable individually.
 |
| Fig.15.12 |
3. But as we can see, there are unsatisfied bonds. They are shown inside small red circles.
• There are a total of four unsatisfied bonds. They are called 'unsatisfied' because, such bonds have only one electron.
• This is shown more clearly in the electron dot diagram in fig.15.13(a) below:
 |
| Fig.15.13 |
♦ 5 green and two red.
• So one more electron is required.
4. Consider fig.15.13(b)
• The two molecules have decided to combine together.
• So the two carbon atoms in the middle will bond together. Thus a larger molecule is formed.
• The result is shown in fig.15.14(a) below:
 |
| Fig.15.14 |
• Even when this larger molecule is formed from two smaller molecules, the outer two carbon atoms still need one electron each. We can represent it as: ㅡ[CH2ㅡCH2]2ㅡ
• 'CH2ㅡCH2' is one ethene molecule. Putting it inside square brackets and giving a subscript '2' indicates that two ethene molecules are combined together inside the square brackets.
• The 'ㅡ' symbol at both ends indicate that, even after the combination, unsatisfied bond exists at the ends
6. Obviously, another two such simple molecules of ethene can be attached at the ends. This is shown by the ellipses in fig.15.8(b).
• The resulting larger molecule can be represented as: ㅡ[CH2ㅡCH2]4ㅡ .
7. In this way a long chain can be formed with a large number of ethene molecules.
• The structure of the chain is shown in fig.15.15 below:
 |
| Fig.15.15 |
♦ Where 'n' is a very large number
Polymerisation is the process in which a large number of simple molecules combine under suitable conditions to form complex molecules.
• The complex molecules thus formed are called polymers.
• The simple molecules which combine in this manner are called monomers.
• We use a number of polymers in our daily life.
♦ Some of them are natural polymers
♦ Others are man-made polymers
■ In the example that we saw above, the monomer is ethene
■ The IUPAC name of the polymer is polyethene
• It is commonly known as polythene.
• It is also known as polyethylene. Since ethylene was the common name for ethene before IUPAC names were implemented.
• It is the most common type of plastic, and is used for making plastic bags, bottles etc.,
■ The IUPAC name of another important polymer is: Poly(1-chloroethene)
• From this IUPAC name, we get the following information:
(i) The monomer is written inside the brackets. It is: 1-chloroethene
(ii) '1-chloroethene' indicates that it is an alkene. In this case, the alkene is obviously ethene.
(iii) One hydrogen atom in the ethene is replaced by a chlorine atom. Hence the name: 1-chloroethene
(iv) So the monomer is: CH2=CHCl. It's structure is shown in fig.15.16(a) below.
• But the double bond between the two carbon atoms breaks and become a single bond.
♦ So it can be represented as: ㅡ[CH2ㅡCHCl]ㅡ
♦ It's structure is shown in fig.15.16(b) below.
• Now it is ready to form the chain. The chain can be represented as: ㅡ[CH2ㅡCHCl]nㅡ
♦ It's structure can be represented as shown in fig.15.16(c) below:
 |
| Fig.15.16 |
• The monomer '1-chloroethene', or simply 'chloroethene', is commonly known as vinylchloride
• So when the monomer vinylchloride becomes a polymer, it's common name will be polyvinylchloride.
• It is popularly known as PVC, and is used for making pipes, electric cable insulation etc.,
■ The IUPAC name of another important polymer is: Poly(1,1,2,2-tetrafluoroethene)
• From this IUPAC name, we get the following information:
(i) The monomer is written inside the brackets. It is: 1,1,2,2-tetrafluoroethene
(ii) '1,1,2,2-tetrafluoroethene' indicates that it is an alkene. In this case, the alkene is obviously ethene.
(iii) One ethene molecule has four hydrogen atoms. All those hydrogen atoms are replaced by fluorine atoms. Hence the name: 1,1,2,2-tetrafluoroethene
(iv) So the monomer is: CF2=CF2. It's structure is shown in fig.15.17(a) below.
• But the double bond between the two carbon atoms breaks and become a single bond.
♦ So it can be represented as: ㅡ[CF2ㅡCF2]ㅡ
♦ It's structure is shown in fig.15.17(b) below.
• Now it is ready to form the chain. The chain can be represented as: ㅡ[CF2ㅡCF2]nㅡ
♦ It's structure can be represented as shown in fig.15.17(c) below:
 |
| Fig.15.17 |
• The monomer 1,1,2,2-tetrafluoroethene, is commonly known simply as tetrafluoroethene
• When the monomer tetrafluoroethene becomes a polymer, that polymer is commonly know as teflon.• It is used for coating the inner surface of non-stick cookware.
■ The IUPAC name of yet another important polymer is: Poly(propene)
• From this IUPAC name, we get the following information:
(i) The monomer is written inside the brackets. It is: propene
(ii) We know that 'propene' is an alkene. It's formula is: CH3ㅡCH=CH2
(iii) It's structure is shown in fig.15.18(a) below.
• But the double bond between the two carbon atoms breaks and become a single bond.
But it cannot be represented as: ㅡ[CH3ㅡCHㅡCH2]ㅡ
Let us analyse the reason:
(i) Till now we have seen monomers with upto two carbon atoms only.
(ii) But our present monomer propene has 3 carbon atoms.
iii) So while writing the structure, the carbon atoms on either sides of the double bond should come in a straight line.
(iv) This is because, the 'unsatisfied bonds' will be starting from the carbon atoms on either sides of the double bond
(v) The third carbon atom should be written above or below the line. This is shown in fig.15.18(b) below:
 |
| Fig.15.18 |
• The propene is commonly known as propylene
• So when the monomer propylene becomes a polymer, it's common name will be polypropylene
• It is used for making table tops and other utensils which require thermal resistance.
■ It is interesting to note that rubber (natural and man-made) is a polymer. The monomer in this case is isoprene
• Isoprene is an alkene having two double bonds. We will see it's polymer structure in higher classes
In the next section, we will see some more reactions.
No comments:
Post a Comment