In the previous section, we saw GMM. In this section we will see the details about 'mole':
We have seen that,
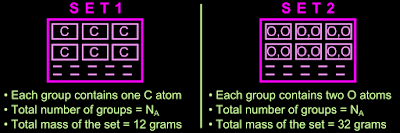
• 1 GAM of any element will contain NA atoms
♦ That is., 6.022×1023 atoms
• 1 GMM of any element/compound will contain NA molecules
♦ That is., 6.022×1023 molecules
■ This 6.022×1023 is a very important number in chemistry. So it is given a special name: mole.
Let us see a comparison:
• Consider a group of apples. If there are 12 apples in that group, we say: A dozen apples
• Consider a group of atoms. If there are 6.022×1023 atoms in that group, we say: A mole atoms
• Consider a group of molecules. If there are 6.022×1023 molecules in that group, we say: A mole molecules
• Consider a group of ions. If there are 6.022×1023 ions in that group, we say: A mole ions
■ While using mole, it is important to specify the name of the particle. That is., whether it is atom, molecule or ion.
Based on the above discussion, a relation can be established between two sides. One side consists of the mole. The other side consists of Avogadro number (NA), GAM and GMM. The fig.10.6 below illustrates the relation:
■ The definition for mole can be written as follows:
The amount of any substance containing 6.022×1023 particles is called one mole (1 mole or 1 mol)
The concept will become more clear when we see some solved examples
Solved example 10.3
A sample of water contains 10,000 water molecules. How many moles of molecules are present in it?
Solution:
1. One mole of molecules will contain 6.022×1023 molecules.
2. So no. of moles in 10000 molecules = 10000⁄(6.022 × 1023)
Solved example 10.4
Find the number of moles in the following:
(i) 1,00,000 CO2 molecules
(ii) 1,00,000 H2 molecules
(iii) 6.022×1023 glucose molecules
Solution (i):
1. One mole of molecules will contain 6.022×1023 molecules.
2. So no. of moles in 1,00,00 molecules = 100000⁄(6.022 × 1023)
That is., 1,00,000 CO2 molecules will contain [100000⁄(6.022 × 1023)] moles
Solution (ii):
1. One mole of molecules will contain 6.022×1023 molecules.
2. So no. of moles in 1,00,00 molecules = 100000⁄(6.022 × 1023) .
That is., 1,00,000 H2 molecules will contain [100000⁄(6.022 × 1023)] moles
Solution (iii):
1. One mole of molecules will contain 6.022×1023 molecules.
2. So no. of moles in 6.022×1023 molecules = (6.022 × 1023)⁄(6.022 × 1023) = 1
That is., 6.022×1023 glucose molecules will contain 1 mole
Solved example 10.5
Find the number of moles of atoms in 160 grams of oxygen
Solution:
1. One mole of atoms will contain 6.022×1023 atoms.
• So no. of moles of atoms = (Total no. of atoms)⁄(6.022 × 1023) .
2. But we are not given 'Total no. of atoms'. We have to calculate it from the given 'total mass'
• Given total mass = 160 g
3. 1 GAM of oxygen will contain 6.022×1023 atoms of oxygen
• 1 GAM of oxygen = 16 g
• No. of GAMs in 160 g = 160⁄16 = 10
4. So no. of atoms in 160 g = 10 × 6.022×1023
5. Substituting this in (1) we get:
No. of moles of atoms = (Total no. of atoms)⁄(6.022 × 1023) = (10×6.022×1023)⁄(6.022×1023) = 10
• We can write the above result in the form of an equation:
Eq.10.1:
No. of moles of atoms/molecules = (Total no. of atoms/molecules)⁄NA
■ Another method using basic information:
1. We have:
No. of atoms × Mass (in grams) of one atom = Given total Mass (in grams)
⇒ No. of atoms = Given total Mass (in grams)⁄Mass (in grams) of one atom.
2. Given total mass = 160 grams
3. Mass (in u) of one atom of oxygen = 16 u
4. But 1 u = 1.6605×10-24 grams (Details here)
So 16 u = 16 × 1.6605×10-24 grams
5. Substituting this value in (1) we get:
No. of atoms = Given total Mass (in grams)⁄Mass (in grams) of one atom
= 160⁄(16×1.6605×10-24) = 10⁄(1.6605×10-24)
6. No. of moles of atoms = No. of atoms⁄(6.022×1023)
= [10⁄(1.6605×10-24)] ÷ 6.022×1023 = [10⁄(1.6605×10-24)] × [1⁄(6.022 × 1023)]
7. But 1⁄(6.022 × 1023) = 1.6605×10-24. So numerator and denominator will cancel each other. We get 10 as the answer
Solved example 10.6
Find the number of moles of molecules in 160 grams of oxygen
Solution:
1. One mole of molecules will contain 6.022×1023 molecules.
• So no. of moles = (Total no. of molecules)⁄(6.022 × 1023) .
2. But we are not given 'Total no. of molecules'. We have to calculate it from the given 'total mass'
• Given total mass = 160 g
3. 1 GMM of oxygen will contain 6.022×1023 molecules of oxygen
• 1 GMM of oxygen = 32 g
• No. of GMMs in 160 g = 160⁄32 = 5
4. So no. of molecules in 160 g = 5 × 6.022×1023
5. Substituting this in (1) we get:
No. of moles = (Total no. of molecules)⁄(6.022 × 1023) = (5×6.022×1023)⁄(6.022×1023) = 5
■ Another method using basic information:
1. We have:
No. of molecules × Mass (in grams) of one molecule = Given total Mass (in grams)
⇒ No. of molecules = Given total Mass (in grams)⁄Mass (in grams) of one molecule .
2. Given total mass = 160 grams
3. Mass (in u) of one molecule of oxygen = 32 u
4. But 1 u = 1.6605×10-24 grams (Details here)
So 32 u = 32 × 1.6605×10-24 grams
5. Substituting this value in (1) we get:
No. of molecules = Given total Mass (in grams)⁄Mass (in grams) of one atom
= 160⁄(32×1.6605×10-24) = 5⁄(1.6605×10-24)
6. No. of moles of molecules = No. of molecules⁄(6.022×1023)
= [5⁄(1.6605×10-24)] ÷ 6.022×1023 = [5⁄(1.6605×10-24)] × [1⁄(6.022 × 1023)]
7. But 1⁄(6.022 × 1023) = 1.6605×10-24. So numerator and denominator will cancel each other. We get 5 as the answer
Solved example 10.7
Find the number of moles of molecules in 220 grams of CO2
Solution:
1. One mole of molecules will contain 6.022×1023 molecules.
• So no. of moles = (Total no. of molecules)⁄(6.022 × 1023)
2. But we are not given 'Total no. of molecules'. We have to calculate it from the given 'total mass'
• Given total mass = 220 g
3. 1 GMM of CO2 will contain 6.022×1023 molecules of CO2
• 1 GMM of CO2 = (1×12) + (2×16) = 12 + 32 = 44 g
• No. of GMMs in 220 g = 220⁄44 = 5
4. So no. of molecules in 220 g = 5 × 6.022×1023
5. Substituting this in (1) we get:
No. of moles = (Total no. of molecules)⁄(6.022 × 1023) = (5×6.022×1023)⁄(6.022×1023) = 5
■ Another method using basic information:
1. We have:
No. of molecules × Mass (in grams) of one molecule = Given total Mass (in grams)
⇒ No. of molecules = Given total Mass (in grams)⁄Mass (in grams) of one molecule .
2. Given total mass = 220 grams
3. Mass (in u) of one molecule of CO2 = (1×12) + (2×16) = 12 + 32 = 44 u
4. But 1 u = 1.6605×10-24 grams (Details here)
So 44 u = 44 × 1.6605×10-24 grams
5. Substituting this value in (1) we get:
No. of molecules = Given total Mass (in grams)⁄Mass (in grams) of one atom
= 220⁄(44×1.6605×10-24) = 5⁄(1.6605×10-24)
6. No. of moles of molecules = No. of molecules⁄(6.022×1023)
= [5⁄(1.6605×10-24)] ÷ 6.022×1023 = [5⁄(1.6605×10-24)] × [1⁄(6.022 × 1023)]
7. But 1⁄(6.022 × 1023) = 1.6605×10-24. So numerator and denominator will cancel each other. We get 5 as the answer
Solved example 10.8
Find
(i) The number of moles of atoms in 700 grams of nitrogen
(ii) The number of moles of molecules in 700 grams of nitrogen
(iii) Number of moles of sugar molecules in 1 kg of sugar (C12H22O11) and the number of moles of carbon atoms present in that 1 kg
(iv) Number of moles in 3.011×1023 carbon atoms
Solution (i):
1. One mole of atoms will contain 6.022×1023 atoms.
• So no. of moles = (Total no. of atoms)⁄(6.022 × 1023) .
2. But we are not given 'Total no. of atoms'. We have to calculate it from the given 'total mass'
• Given total mass = 700 g
3. 1 GAM of nitrogen will contain 6.022×1023 atoms of nitrogen
• 1 GAM of nitrogen = 14 g
• No. of GAMs in 700 g = 700⁄14 = 50
4. So no. of atoms in 700 g = 50 × 6.022×1023
5. Substituting this in (1) we get:
No. of moles = (Total no. of atoms)⁄(6.022 × 1023) = (50×6.022×1023)⁄(6.022×1023) = 50
Solution (ii):
1. One mole of molecules will contain 6.022×1023 molecules.
• So no. of moles = (Total no. of molecules)⁄(6.022 × 1023)
2. But we are not given 'Total no. of molecules'. We have to calculate it from the given 'total mass'
• Given total mass = 700 g
3. 1 GMM of nitrogen will contain 6.022×1023 molecules of nitrogen
• 1 GMM of nitrogen = 28 g
• No. of GMMs in 700 g = 700⁄28 = 25
4. So no. of molecules in 700 g = 25 × 6.022×1023
5. Substituting this in (1) we get:
No. of moles = (Total no. of molecules)⁄(6.022 × 1023) = (25×6.022×1023)⁄(6.022×1023) = 25
Solution (iii):
1. One mole of molecules will contain 6.022×1023 molecules.
• So no. of moles = (Total no. of molecules)⁄(6.022 × 1023)
2. But we are not given 'Total no. of molecules'. We have to calculate it from the given 'total mass'
• Given total mass = 1 kg = 1000 g
3. 1 GMM of C12H22O11 will contain 6.022×1023 molecules of C12H22O11
• 1 GMM of C12H22O11 = (12×12) + (22×1) + (11×16) = 144 + 22 + 176 = 342 g
• No. of GMMs in 1000 g = 1000⁄342 = 2.92
4. So no. of molecules in 1000 g = 2.92 × 6.022×1023
5. Substituting this in (1) we get:
No. of moles = (Total no. of molecules)⁄(6.022 × 1023) = (2.92×6.022×1023)⁄(6.022×1023) = 2.92
6. From (4) we get the total number of sugar molecules as 2.92 × 6.022×1023.
7. Each of these molecules will contain 12 carbon atoms.
So total number of carbon atoms in the 1000 grams of sugar = 12 × 2.92 × 6.022×1023.
8. So no. of moles of carbon atoms = (Total no. of atoms)⁄(6.022 × 1023)
= (12×2.92×6.022×1023)⁄(6.022×1023) = 12×2.92 = 35.04 moles
Solution (iv):
1. One mole of atoms will contain 6.022×1023 atoms.
• So no. of moles = (Total no. of atoms)⁄(6.022 × 1023)
2. We are given 'Total no. of atoms'. It is 3.011×1023.
3. So no. of moles of carbon atoms = (Total no. of atoms)⁄(6.022 × 1023)
= (3.011×1023)⁄(6.022×1023) = 0.5 moles
So we now know how to convert mass into 'number of moles'. We can also convert a given number of atoms/molecules into 'number of moles'. In the next section we will see the reverse. That is., conversion of 'number of moles' into mass.
We have seen that,
• 1 GAM of any element will contain NA atoms
♦ That is., 6.022×1023 atoms
• 1 GMM of any element/compound will contain NA molecules
♦ That is., 6.022×1023 molecules
■ This 6.022×1023 is a very important number in chemistry. So it is given a special name: mole.
Let us see a comparison:
• Consider a group of apples. If there are 12 apples in that group, we say: A dozen apples
• Consider a group of atoms. If there are 6.022×1023 atoms in that group, we say: A mole atoms
• Consider a group of molecules. If there are 6.022×1023 molecules in that group, we say: A mole molecules
• Consider a group of ions. If there are 6.022×1023 ions in that group, we say: A mole ions
■ While using mole, it is important to specify the name of the particle. That is., whether it is atom, molecule or ion.
 |
| Fig.10.6 |
The amount of any substance containing 6.022×1023 particles is called one mole (1 mole or 1 mol)
Solved example 10.3
A sample of water contains 10,000 water molecules. How many moles of molecules are present in it?
Solution:
1. One mole of molecules will contain 6.022×1023 molecules.
2. So no. of moles in 10000 molecules = 10000⁄(6.022 × 1023)
Solved example 10.4
Find the number of moles in the following:
(i) 1,00,000 CO2 molecules
(ii) 1,00,000 H2 molecules
(iii) 6.022×1023 glucose molecules
Solution (i):
1. One mole of molecules will contain 6.022×1023 molecules.
2. So no. of moles in 1,00,00 molecules = 100000⁄(6.022 × 1023)
That is., 1,00,000 CO2 molecules will contain [100000⁄(6.022 × 1023)] moles
Solution (ii):
1. One mole of molecules will contain 6.022×1023 molecules.
2. So no. of moles in 1,00,00 molecules = 100000⁄(6.022 × 1023) .
That is., 1,00,000 H2 molecules will contain [100000⁄(6.022 × 1023)] moles
Solution (iii):
1. One mole of molecules will contain 6.022×1023 molecules.
2. So no. of moles in 6.022×1023 molecules = (6.022 × 1023)⁄(6.022 × 1023) = 1
That is., 6.022×1023 glucose molecules will contain 1 mole
Solved example 10.5
Find the number of moles of atoms in 160 grams of oxygen
Solution:
1. One mole of atoms will contain 6.022×1023 atoms.
• So no. of moles of atoms = (Total no. of atoms)⁄(6.022 × 1023) .
2. But we are not given 'Total no. of atoms'. We have to calculate it from the given 'total mass'
• Given total mass = 160 g
3. 1 GAM of oxygen will contain 6.022×1023 atoms of oxygen
• 1 GAM of oxygen = 16 g
• No. of GAMs in 160 g = 160⁄16 = 10
4. So no. of atoms in 160 g = 10 × 6.022×1023
5. Substituting this in (1) we get:
No. of moles of atoms = (Total no. of atoms)⁄(6.022 × 1023) = (10×6.022×1023)⁄(6.022×1023) = 10
• We can write the above result in the form of an equation:
Eq.10.1:
No. of moles of atoms/molecules = (Total no. of atoms/molecules)⁄NA
■ Another method using basic information:
1. We have:
No. of atoms × Mass (in grams) of one atom = Given total Mass (in grams)
⇒ No. of atoms = Given total Mass (in grams)⁄Mass (in grams) of one atom.
2. Given total mass = 160 grams
3. Mass (in u) of one atom of oxygen = 16 u
4. But 1 u = 1.6605×10-24 grams (Details here)
So 16 u = 16 × 1.6605×10-24 grams
5. Substituting this value in (1) we get:
No. of atoms = Given total Mass (in grams)⁄Mass (in grams) of one atom
= 160⁄(16×1.6605×10-24) = 10⁄(1.6605×10-24)
6. No. of moles of atoms = No. of atoms⁄(6.022×1023)
= [10⁄(1.6605×10-24)] ÷ 6.022×1023 = [10⁄(1.6605×10-24)] × [1⁄(6.022 × 1023)]
7. But 1⁄(6.022 × 1023) = 1.6605×10-24. So numerator and denominator will cancel each other. We get 10 as the answer
Solved example 10.6
Find the number of moles of molecules in 160 grams of oxygen
Solution:
1. One mole of molecules will contain 6.022×1023 molecules.
• So no. of moles = (Total no. of molecules)⁄(6.022 × 1023) .
2. But we are not given 'Total no. of molecules'. We have to calculate it from the given 'total mass'
• Given total mass = 160 g
3. 1 GMM of oxygen will contain 6.022×1023 molecules of oxygen
• 1 GMM of oxygen = 32 g
• No. of GMMs in 160 g = 160⁄32 = 5
4. So no. of molecules in 160 g = 5 × 6.022×1023
5. Substituting this in (1) we get:
No. of moles = (Total no. of molecules)⁄(6.022 × 1023) = (5×6.022×1023)⁄(6.022×1023) = 5
■ Another method using basic information:
1. We have:
No. of molecules × Mass (in grams) of one molecule = Given total Mass (in grams)
⇒ No. of molecules = Given total Mass (in grams)⁄Mass (in grams) of one molecule .
2. Given total mass = 160 grams
3. Mass (in u) of one molecule of oxygen = 32 u
4. But 1 u = 1.6605×10-24 grams (Details here)
So 32 u = 32 × 1.6605×10-24 grams
5. Substituting this value in (1) we get:
No. of molecules = Given total Mass (in grams)⁄Mass (in grams) of one atom
= 160⁄(32×1.6605×10-24) = 5⁄(1.6605×10-24)
6. No. of moles of molecules = No. of molecules⁄(6.022×1023)
= [5⁄(1.6605×10-24)] ÷ 6.022×1023 = [5⁄(1.6605×10-24)] × [1⁄(6.022 × 1023)]
7. But 1⁄(6.022 × 1023) = 1.6605×10-24. So numerator and denominator will cancel each other. We get 5 as the answer
Solved example 10.7
Find the number of moles of molecules in 220 grams of CO2
Solution:
1. One mole of molecules will contain 6.022×1023 molecules.
• So no. of moles = (Total no. of molecules)⁄(6.022 × 1023)
2. But we are not given 'Total no. of molecules'. We have to calculate it from the given 'total mass'
• Given total mass = 220 g
3. 1 GMM of CO2 will contain 6.022×1023 molecules of CO2
• 1 GMM of CO2 = (1×12) + (2×16) = 12 + 32 = 44 g
• No. of GMMs in 220 g = 220⁄44 = 5
4. So no. of molecules in 220 g = 5 × 6.022×1023
5. Substituting this in (1) we get:
No. of moles = (Total no. of molecules)⁄(6.022 × 1023) = (5×6.022×1023)⁄(6.022×1023) = 5
■ Another method using basic information:
1. We have:
No. of molecules × Mass (in grams) of one molecule = Given total Mass (in grams)
⇒ No. of molecules = Given total Mass (in grams)⁄Mass (in grams) of one molecule .
2. Given total mass = 220 grams
3. Mass (in u) of one molecule of CO2 = (1×12) + (2×16) = 12 + 32 = 44 u
4. But 1 u = 1.6605×10-24 grams (Details here)
So 44 u = 44 × 1.6605×10-24 grams
5. Substituting this value in (1) we get:
No. of molecules = Given total Mass (in grams)⁄Mass (in grams) of one atom
= 220⁄(44×1.6605×10-24) = 5⁄(1.6605×10-24)
6. No. of moles of molecules = No. of molecules⁄(6.022×1023)
= [5⁄(1.6605×10-24)] ÷ 6.022×1023 = [5⁄(1.6605×10-24)] × [1⁄(6.022 × 1023)]
7. But 1⁄(6.022 × 1023) = 1.6605×10-24. So numerator and denominator will cancel each other. We get 5 as the answer
Solved example 10.8
Find
(i) The number of moles of atoms in 700 grams of nitrogen
(ii) The number of moles of molecules in 700 grams of nitrogen
(iii) Number of moles of sugar molecules in 1 kg of sugar (C12H22O11) and the number of moles of carbon atoms present in that 1 kg
(iv) Number of moles in 3.011×1023 carbon atoms
Solution (i):
1. One mole of atoms will contain 6.022×1023 atoms.
• So no. of moles = (Total no. of atoms)⁄(6.022 × 1023) .
2. But we are not given 'Total no. of atoms'. We have to calculate it from the given 'total mass'
• Given total mass = 700 g
3. 1 GAM of nitrogen will contain 6.022×1023 atoms of nitrogen
• 1 GAM of nitrogen = 14 g
• No. of GAMs in 700 g = 700⁄14 = 50
4. So no. of atoms in 700 g = 50 × 6.022×1023
5. Substituting this in (1) we get:
No. of moles = (Total no. of atoms)⁄(6.022 × 1023) = (50×6.022×1023)⁄(6.022×1023) = 50
Solution (ii):
1. One mole of molecules will contain 6.022×1023 molecules.
• So no. of moles = (Total no. of molecules)⁄(6.022 × 1023)
2. But we are not given 'Total no. of molecules'. We have to calculate it from the given 'total mass'
• Given total mass = 700 g
3. 1 GMM of nitrogen will contain 6.022×1023 molecules of nitrogen
• 1 GMM of nitrogen = 28 g
• No. of GMMs in 700 g = 700⁄28 = 25
4. So no. of molecules in 700 g = 25 × 6.022×1023
5. Substituting this in (1) we get:
No. of moles = (Total no. of molecules)⁄(6.022 × 1023) = (25×6.022×1023)⁄(6.022×1023) = 25
Solution (iii):
1. One mole of molecules will contain 6.022×1023 molecules.
• So no. of moles = (Total no. of molecules)⁄(6.022 × 1023)
2. But we are not given 'Total no. of molecules'. We have to calculate it from the given 'total mass'
• Given total mass = 1 kg = 1000 g
3. 1 GMM of C12H22O11 will contain 6.022×1023 molecules of C12H22O11
• 1 GMM of C12H22O11 = (12×12) + (22×1) + (11×16) = 144 + 22 + 176 = 342 g
• No. of GMMs in 1000 g = 1000⁄342 = 2.92
4. So no. of molecules in 1000 g = 2.92 × 6.022×1023
5. Substituting this in (1) we get:
No. of moles = (Total no. of molecules)⁄(6.022 × 1023) = (2.92×6.022×1023)⁄(6.022×1023) = 2.92
6. From (4) we get the total number of sugar molecules as 2.92 × 6.022×1023.
7. Each of these molecules will contain 12 carbon atoms.
So total number of carbon atoms in the 1000 grams of sugar = 12 × 2.92 × 6.022×1023.
8. So no. of moles of carbon atoms = (Total no. of atoms)⁄(6.022 × 1023)
= (12×2.92×6.022×1023)⁄(6.022×1023) = 12×2.92 = 35.04 moles
Solution (iv):
1. One mole of atoms will contain 6.022×1023 atoms.
• So no. of moles = (Total no. of atoms)⁄(6.022 × 1023)
2. We are given 'Total no. of atoms'. It is 3.011×1023.
3. So no. of moles of carbon atoms = (Total no. of atoms)⁄(6.022 × 1023)
= (3.011×1023)⁄(6.022×1023) = 0.5 moles
So we now know how to convert mass into 'number of moles'. We can also convert a given number of atoms/molecules into 'number of moles'. In the next section we will see the reverse. That is., conversion of 'number of moles' into mass.