In the previous section, we saw the equilibrium state of a chemical reaction. In this section we will learn about the factors which affect the Equilibrium.
• Ans: The concentration of that reactant must decrease back to the previous level. For that, the rate of forward reaction will increase, so as to convert the extra reactant to product.
Consider the example: N2 (g) + 3H2 (g) ⇌ 2NH3 (g)
■ If we add some nitrogen to the system, what will happen?
Ans: The rate of forward reaction will increase so as to convert more nitrogen into ammonia. As a result, production of ammonia will increase.
■ If we add some ammonia to the system, what will happen?
Ans: The rate of backward reaction will increase so as to convert more ammonia back into nitrogen and hydrogen.
■ If we remove ammonia continuously from the system, what will happen?
Ans: The rate of forward reaction will increase so as to produce more ammonia
• The above discussion gives us an idea about the influence of concentrations on chemical equilibrium.
• Influence of pressure is felt in reactions involving gases. It can be analyzed using an example:
• Consider the reaction between nitrogen and hydrogen which gives ammonia as the product. The balanced chemical equation is:
N2 (g) + 3H2 (g) ⇌ 2NH3 (g)
• One molecule of nitrogen reacts with 3 molecules of hydrogen to produce two molecules of ammonia.
• So, if we take NA (Avogadro number) of molecules of nitrogen, we must take 3NA molecules of hydrogen. The result will be 2NA molecules of ammonia.
• It is clear that, there are greater number of molecules on the reactant side than in the product side.
• In gaseous systems, larger number of molecules will produce greater pressure.
Consider a state of equilibrium in the manufacture of ammonia.
■ If at this equilibrium we increase the pressure, what will happen?
Ans: The system will try to reduce that pressure. For that, the number of molecules must decrease.
• For a decrease in the number of molecules, more quantities of the product should be formed. Because, in this reaction, number of molecules on the product side is lower.
• So rate of the forward reaction will increase
■ If at this equilibrium we decrease the pressure, what will happen?
Ans: The system will try to increase the pressure. For that, the number of molecules must increase.
• For an increase in the number of molecules, more quantities of the reactants should be formed. Because, in this reaction, number of molecules on the reactants side is higher.
• So rate of the backward reaction will increase
■ In the manufacture of ammonia, why is a pressure of 200 - 900 atm used?
Ans: In the manufacture of ammonia, high pressure is used so that, the rate of the forward reaction will increase and more ammonia will be formed.
Is an increase in pressure always helpful? Let us see another example:
Consider the balanced equation:
H2 (g) + I2 (g) ⇌ 2HI (g)
• One molecule of hydrogen reacts with 1 molecule of iodine to produce two molecules of hydrogen iodide.
• So, if we take NA (Avogadro number) of molecules of hydrogen, we must take NA molecules of iodine. The result will be 2NA molecules of hydrogen iodide.
• It is clear that, there are equal number of molecules on the reactant side and in the product side.
■ If there is no change in the number of reactant or product molecules as a result of forward and backward reactions, in gaseous reactions, pressure will not have any effect on the equilibrium state.
• Endothermic reactions are those in which heat is absorbed during the reaction
• Exothermic reactions are those in which heat is liberated during the reaction
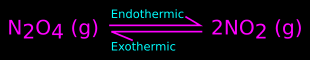
Consider the equation of a reversible reaction:
1. In the forward reaction, N2O4 changes to NO2. This forward reaction is endothermic. That is., if this forward reaction is to take place, we have to supply heat
2. In the backward reaction, NO2 changes to N2O4. This backward reaction is exothermic. That is., when this backward reaction takes place, we will get heat.
3. Consider a system in which the above reaction is at equilibrium.
• If this system at equilibrium is placed in ice, the temperature will be lowered.
• The system will then try to increase the temperature by producing more heat.
• To produce heat, exothermic reaction must take place.
• So rate of the backward reaction increases. Thus So more NO2 will be converted to N2O4.
4. Consider a system in which the above reaction is at equilibrium.
• If this system at equilibrium is placed in hot water, the temperature will be increased.
• The system will then try to decrease the temperature.
• To reduce temperature, heat must be absorbed. For heat to get absorbed, endothermic reaction must take place.
• So rate of the forward reaction increases. Thus So more N2O4 will be converted to NO2.
■ Note that nitric acid (HNO3) is a very corrosive acid and Nitrogen dioxide (NO2) is a poisonous gas. All safety precautions should be taken while doing experiments.
Let us now see the effect of temperature in the manufacture of ammonia.
1. We know that ammonia is manufactured by Haber process. It is a reversible reaction. It can be represented by the following balanced equation:
N2 (g) + 3H2 (g) ⇌ 2NH3 (g)
2. Here the forward reaction is exothermic in nature. That is., the forward reaction releases heat. So we can rewrite the equation as:
N2 (g) + 3H2 (g) ⇌ 2NH3 (g) + Heat
3. If we increase the temperature, the system will try to reduce it.
• For that, less heat must be produced. So the rate of backward reaction will increase. We will be getting more nitrogen and hydrogen instead of ammonia. So it is not advisable to increase the temperature.
4. If we cannot increase the temperature, can we decrease it?
• Reducing the temperature to low values has adverse effects. When the temperature is low, the rates of both forward and backward reactions become very low. (This is because, the molecules must have a minimum amount of energy for the collisions to take place)
• As a result, the system will take more time to reach equilibrium. Hence in the manufacture of ammonia, 450o c is taken as optimum temperature.
• In reversible reactions, there is forward as well as backward reactions taking place.
• A positive catalyst will increase the rate of both forward and backward reactions. So equilibrium is reached in a lesser time.
• Once equilibrium is reached, the rates of forward or backward reactions can be controlled by altering the other factors like temperature, pressure etc.,
• So, in many reversible reactions, positive catalyst is used at the beginning of the reaction.
The French scientist Henry-Louis Le Chatelier put forward a principle regarding the effects of various factors on chemical equilibrium.
Le Chatelier's Principle:
When the concentration, pressure or temperature of a system at equilibrium is changed, the system will readjust itself so as to nullify the effect of that change. As a result of the readjustment, the system will attain a new state of equilibrium
Now we will see a solved example:
• The system will try to nullify the effect of increase in concentration of a reactant by increasing the rate of the forward reaction.
• So more products will be formed
2. Increase in pressure:
• From the balanced equation, it is clear that, there are greater number of molecules on the reactant side than in the product side.
• In gaseous systems, larger number of molecules will produce greater pressure.
• The system will increase the rate of the forward reaction. So the number of molecules will become less and pressure will become low.
• Thus the effect of increase in pressure will be nullified
3. Maintains temperature at optimum level
• From the given balanced equation it is clear that, the forward reaction is exothermic
• If we increase the temperature, the system will try to nullify it by increasing the rate of the backward reaction. In that case we will get lesser quantities of the product.
• On the other hand, a decrease in temperature is also not advisable. Because, the molecules need a minimum amount of energy for the collisions to take place.
• So, if we maintain an optimum temperature, we will get more products
4. Catalyst V2O5 is added
• The catalyst increases the rate of both forward and backward reactions. So it helps to achieve equilibrium in a lesser duration of time.
5. SO3 is removed
• When SO3 is removed, concentration of product decreases. To nullify the effect of this 'decrease in concentration', more SO3 has to be produced. For that, the rate of forward reaction increases
Solved example 11.2
(a) In which of the following reactions does the change in pressure not influence equilibrium? What is the reason?
(i) H2 (g) + I2 (g) ⇌ 2HI (g)
(ii) N2 (g) + 3H2 (g) ⇌ 2NH3 (g)
(b) What is the use of applying high pressure duriing the formation of ammonia from nitrogen and hydrogen?
Solution:
(a) In reaction (i), increase or decrease in pressure will not influence equilibrium. Because, the number of molecules in the reactants side and products side are equal
(b) If we increase the pressure, the system will try to reduce that pressure. For that, the number of molecules must decrease.
• For a decrease in the number of molecules, more quantities of the product should be formed. Because, in this reaction, number of molecules on the product side is lower.
• So rate of the forward reaction will increase and more ammonia will be formed.
We have completed the present discussion on Chemical equilibrium. In the next chapter, we will see Reactivity Series and Electrochemistry.
Influence of concentration on Chemical equilibrium
■ Consider a system in equilibrium. If some quantities of a reactant is added to the system, what will happen?• Ans: The concentration of that reactant must decrease back to the previous level. For that, the rate of forward reaction will increase, so as to convert the extra reactant to product.
Consider the example: N2 (g) + 3H2 (g) ⇌ 2NH3 (g)
■ If we add some nitrogen to the system, what will happen?
Ans: The rate of forward reaction will increase so as to convert more nitrogen into ammonia. As a result, production of ammonia will increase.
■ If we add some ammonia to the system, what will happen?
Ans: The rate of backward reaction will increase so as to convert more ammonia back into nitrogen and hydrogen.
■ If we remove ammonia continuously from the system, what will happen?
Ans: The rate of forward reaction will increase so as to produce more ammonia
• Effects of change in concentrations in a system producing ammonia can be written in a tabular form as follows:
| Action | Change of Concentration | Change in rate |
|---|---|---|
| More hydrogen is added | Increases the concentration of the reactant | Rate of forward reaction increases |
| More ammonia is added | Increases the concentration of the product | Rate of backward reaction increases |
| Ammonia is removed | Decreases the concentration of the product | Rate of forward reaction increases |
| More nitrogen is added | Increases the concentration of the reactant | Rate of forward reaction increases |
Influence of pressure on Chemical equilibrium
• Influence of pressure is felt in reactions involving gases. It can be analyzed using an example:
• Consider the reaction between nitrogen and hydrogen which gives ammonia as the product. The balanced chemical equation is:
N2 (g) + 3H2 (g) ⇌ 2NH3 (g)
• One molecule of nitrogen reacts with 3 molecules of hydrogen to produce two molecules of ammonia.
• So, if we take NA (Avogadro number) of molecules of nitrogen, we must take 3NA molecules of hydrogen. The result will be 2NA molecules of ammonia.
• It is clear that, there are greater number of molecules on the reactant side than in the product side.
• In gaseous systems, larger number of molecules will produce greater pressure.
Consider a state of equilibrium in the manufacture of ammonia.
■ If at this equilibrium we increase the pressure, what will happen?
Ans: The system will try to reduce that pressure. For that, the number of molecules must decrease.
• For a decrease in the number of molecules, more quantities of the product should be formed. Because, in this reaction, number of molecules on the product side is lower.
• So rate of the forward reaction will increase
■ If at this equilibrium we decrease the pressure, what will happen?
Ans: The system will try to increase the pressure. For that, the number of molecules must increase.
• For an increase in the number of molecules, more quantities of the reactants should be formed. Because, in this reaction, number of molecules on the reactants side is higher.
• So rate of the backward reaction will increase
■ In the manufacture of ammonia, why is a pressure of 200 - 900 atm used?
Ans: In the manufacture of ammonia, high pressure is used so that, the rate of the forward reaction will increase and more ammonia will be formed.
Is an increase in pressure always helpful? Let us see another example:
Consider the balanced equation:
H2 (g) + I2 (g) ⇌ 2HI (g)
• One molecule of hydrogen reacts with 1 molecule of iodine to produce two molecules of hydrogen iodide.
• So, if we take NA (Avogadro number) of molecules of hydrogen, we must take NA molecules of iodine. The result will be 2NA molecules of hydrogen iodide.
• It is clear that, there are equal number of molecules on the reactant side and in the product side.
■ If there is no change in the number of reactant or product molecules as a result of forward and backward reactions, in gaseous reactions, pressure will not have any effect on the equilibrium state.
Influence of temperature on equilibrium
We have seen endothermic reactions and exothermic reactions in our earlier classes.• Endothermic reactions are those in which heat is absorbed during the reaction
• Exothermic reactions are those in which heat is liberated during the reaction
Consider the equation of a reversible reaction:
1. In the forward reaction, N2O4 changes to NO2. This forward reaction is endothermic. That is., if this forward reaction is to take place, we have to supply heat
2. In the backward reaction, NO2 changes to N2O4. This backward reaction is exothermic. That is., when this backward reaction takes place, we will get heat.
3. Consider a system in which the above reaction is at equilibrium.
• If this system at equilibrium is placed in ice, the temperature will be lowered.
• The system will then try to increase the temperature by producing more heat.
• To produce heat, exothermic reaction must take place.
• So rate of the backward reaction increases. Thus So more NO2 will be converted to N2O4.
4. Consider a system in which the above reaction is at equilibrium.
• If this system at equilibrium is placed in hot water, the temperature will be increased.
• The system will then try to decrease the temperature.
• To reduce temperature, heat must be absorbed. For heat to get absorbed, endothermic reaction must take place.
• So rate of the forward reaction increases. Thus So more N2O4 will be converted to NO2.
■ Note that nitric acid (HNO3) is a very corrosive acid and Nitrogen dioxide (NO2) is a poisonous gas. All safety precautions should be taken while doing experiments.
Let us now see the effect of temperature in the manufacture of ammonia.
1. We know that ammonia is manufactured by Haber process. It is a reversible reaction. It can be represented by the following balanced equation:
N2 (g) + 3H2 (g) ⇌ 2NH3 (g)
2. Here the forward reaction is exothermic in nature. That is., the forward reaction releases heat. So we can rewrite the equation as:
N2 (g) + 3H2 (g) ⇌ 2NH3 (g) + Heat
3. If we increase the temperature, the system will try to reduce it.
• For that, less heat must be produced. So the rate of backward reaction will increase. We will be getting more nitrogen and hydrogen instead of ammonia. So it is not advisable to increase the temperature.
4. If we cannot increase the temperature, can we decrease it?
• Reducing the temperature to low values has adverse effects. When the temperature is low, the rates of both forward and backward reactions become very low. (This is because, the molecules must have a minimum amount of energy for the collisions to take place)
• As a result, the system will take more time to reach equilibrium. Hence in the manufacture of ammonia, 450o c is taken as optimum temperature.
Influence of catalyst on equilibrium
• We know that Positive catalysts are those substances which increase the rate of a reaction.• In reversible reactions, there is forward as well as backward reactions taking place.
• A positive catalyst will increase the rate of both forward and backward reactions. So equilibrium is reached in a lesser time.
• Once equilibrium is reached, the rates of forward or backward reactions can be controlled by altering the other factors like temperature, pressure etc.,
• So, in many reversible reactions, positive catalyst is used at the beginning of the reaction.
The French scientist Henry-Louis Le Chatelier put forward a principle regarding the effects of various factors on chemical equilibrium.
Le Chatelier's Principle:
When the concentration, pressure or temperature of a system at equilibrium is changed, the system will readjust itself so as to nullify the effect of that change. As a result of the readjustment, the system will attain a new state of equilibrium
Now we will see a solved example:
Solved example 11.1
The manufacture of sulphuric acid by contact process has different stages. The chemical equation of one such stage is:
2SO2 (g) + O2 (g) ⇌ 2SO3 (g) + heat
■ Identify how the factors given below influence this reaction
• Increase in the amount of oxygen
• Increase in pressure
• Maintains the temperature at optimum level
• Catalyst (V2O5) is added
• SO3 is removed
Solution:
1. Increase in the amount of oxygen:
• Oxygen is one of the reactants.• The system will try to nullify the effect of increase in concentration of a reactant by increasing the rate of the forward reaction.
• So more products will be formed
2. Increase in pressure:
• From the balanced equation, it is clear that, there are greater number of molecules on the reactant side than in the product side.
• In gaseous systems, larger number of molecules will produce greater pressure.
• The system will increase the rate of the forward reaction. So the number of molecules will become less and pressure will become low.
• Thus the effect of increase in pressure will be nullified
3. Maintains temperature at optimum level
• From the given balanced equation it is clear that, the forward reaction is exothermic
• If we increase the temperature, the system will try to nullify it by increasing the rate of the backward reaction. In that case we will get lesser quantities of the product.
• On the other hand, a decrease in temperature is also not advisable. Because, the molecules need a minimum amount of energy for the collisions to take place.
• So, if we maintain an optimum temperature, we will get more products
4. Catalyst V2O5 is added
• The catalyst increases the rate of both forward and backward reactions. So it helps to achieve equilibrium in a lesser duration of time.
5. SO3 is removed
• When SO3 is removed, concentration of product decreases. To nullify the effect of this 'decrease in concentration', more SO3 has to be produced. For that, the rate of forward reaction increases
Solved example 11.2
(a) In which of the following reactions does the change in pressure not influence equilibrium? What is the reason?
(i) H2 (g) + I2 (g) ⇌ 2HI (g)
(ii) N2 (g) + 3H2 (g) ⇌ 2NH3 (g)
(b) What is the use of applying high pressure duriing the formation of ammonia from nitrogen and hydrogen?
Solution:
(a) In reaction (i), increase or decrease in pressure will not influence equilibrium. Because, the number of molecules in the reactants side and products side are equal
(b) If we increase the pressure, the system will try to reduce that pressure. For that, the number of molecules must decrease.
• For a decrease in the number of molecules, more quantities of the product should be formed. Because, in this reaction, number of molecules on the product side is lower.
• So rate of the forward reaction will increase and more ammonia will be formed.
We have completed the present discussion on Chemical equilibrium. In the next chapter, we will see Reactivity Series and Electrochemistry.
No comments:
Post a Comment